Astrocyte
2022-01-12 links: reference:
- Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation
- Sorting Out Astrocyte Physiology from Pharmacology
- Astrocyte calcium signaling: the third wave
Astrocyte #
The name comes from their starry $\star$ shape… which might be a fake meme.
-
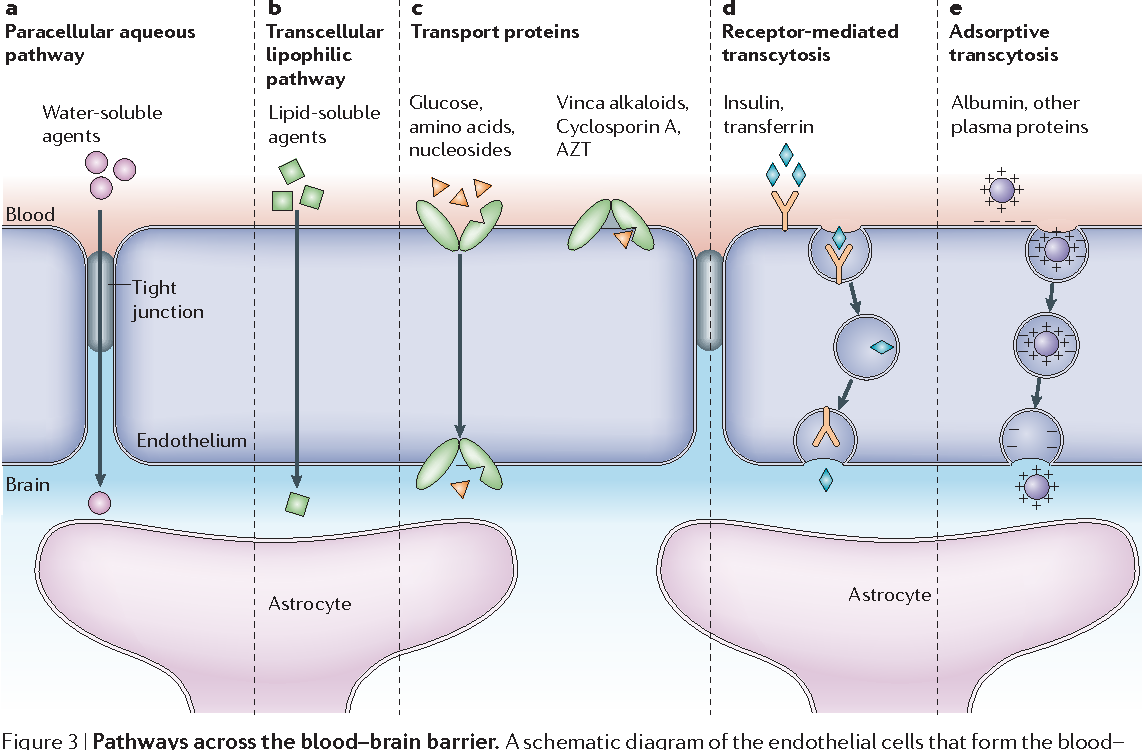
- ‘Water-soluble agents’ would include K+, which astrocytes have a high resting conductance for. It takes them up to dissipate them.
Type of Glia - making up 20-40% of them, are the most numerous cell type in the brain, and the major source of Cholesterol in the CNS.
- Subtypes are Müller cells (found in retina),
- Protoplasmic astrocytes: present in gray matter; profuse branching: a single astrocyte’s processes make contact with thousands of synapses.
- Fibrous astrocytes: white matter; little branching. Mainly to repair.
- Human astrocytes are 20x larger than those in rodents, making contact with 10x the number of synapses.
Function #
- SDF-1 is the natural ligand for chemokine receptor CXCR4 which is located on astrocytes. Activation results in extracellular release of TNF-α which interacts with its cell-surface receptor on astrocytes resulting in generation of PGE2. PGE2 then causes elevation of intracellular Ca2+ resulting in glutamate release from the astrocytes.
-
Astrocytes (2002)
- Mitochondrial/peripheral-type benzodiazepine receptors are extremely abundant in steroidogenic cells. MBR ligands stimulate progesterone synthesis by isolated mitochondria due to MBR translocation of cholesterol from outside to in the Membrane.
- Take up excess Ammonia. They swell during hyperammonemia:
Neurotransmission #
The term “tripartite synapse” is the name of the game here.
- Has Neuroreceptorss. Excitation via Glutamate, binding to nAChR, adenosine, ATP, GABA, histamine, NE induces Ca2+ oscillations (internal and between eachother).
- Mainly via IP3 and Ca2+ through gap functions, and P27.
- Ca2+ oscillations stimulates Phospholipase A2, resulting in accumulation of Arachidonic Acid.
-
A role for the arachidonic acid cascade in fast synaptic modulation: ion channels and transmitter uptake systems as target proteins
- Arachidonic Acid is released upon stimulation of NMDARs. AA inhibits the rate of Glutamate uptake in neuronal synaptic terminals and astrocytes.
- Neither biotransformation nor membrane incorporation are required for AA to exert this effect.
- Mechanism is possibly due to direct interaction with transporters or its lipidic microenvironment right outside the cell membrane.
- PUFAs mimic arachidonate with a rank of potency parallel to the degree of unsaturation.
- Neither biotransformation nor membrane incorporation are required for AA to exert this effect.
- Arachidonic Acid is released upon stimulation of NMDARs. AA inhibits the rate of Glutamate uptake in neuronal synaptic terminals and astrocytes.
-
A role for the arachidonic acid cascade in fast synaptic modulation: ion channels and transmitter uptake systems as target proteins
- Releases GABA via Best1 which is Ca2+-activated.
Glutamate #
- For uptake, they contain various mGluR, EAAT.
- Glutamine Synthetase is found primarily in astrocytes. Additionally, Alanine Aminotransferase is present. The glutamine is transported back to the presynaptic membrane via SNAT. Then of course it’s back to GLU life after glutaminase.
- Release gliotransmitters (Glutamate, ATP, D-Serine, et al) in a Ca2+-dependent manner, and via the Cystine-Glutamate Antiporter
- Targets mGluR, Kainate Receptors, and especially NMDARs.
- Increases likelihood of release and frequency of AMPAR-dependent postynaptic currents. R
- They may be adjacent to eNMDARs, so you gotta be careful.
Inflammation #
-
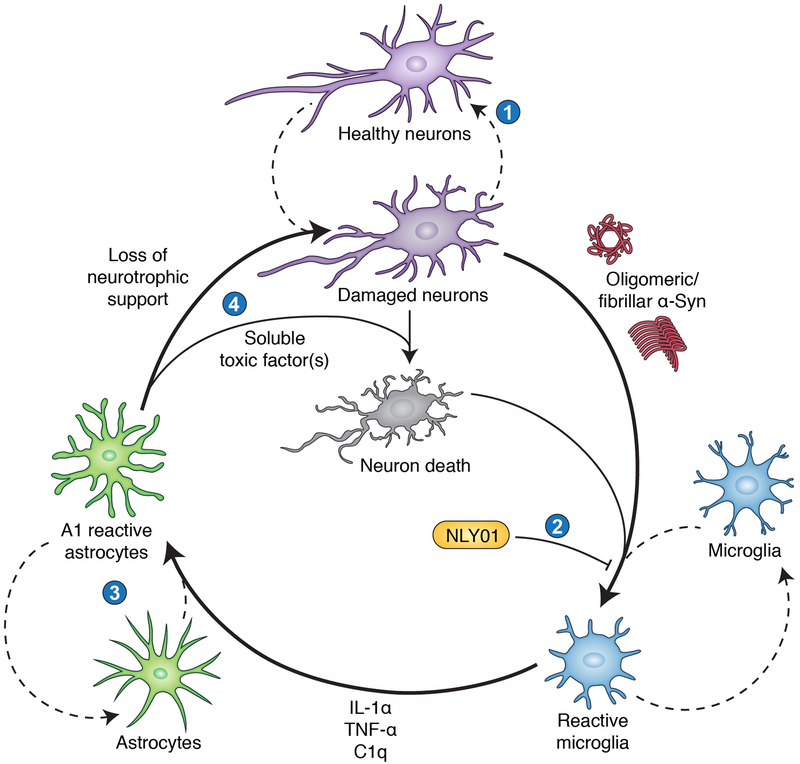
Just like M1/M2 microglia, there are A1 (inflammatory) and A2 (protective) “reactive” astrocytes. Apparently, the toxic factor they actually secrete is unknown. But I don’t see why it couldn’t simply be the loss of support that is the “toxin”, especially considering A1 induction is microglia-dependent.
-
I think this started it all: Neurotoxic reactive astrocytes are induced by activated microglia (Liddelow, Clarke, et al. 2017)
- Obviously, they help drive neuron/oligodendrocyte death in all the neurodegenerative disorders including Multiple Sclerosis.
- Inhibition of A1 prevents death of axotomized neurons after CNS injury. Indeed they lose most normal astrocyte functions.
- Including no longer promoting phagocytosis of synapses/myelin debris.
- A2 is promoted by ischemia.
-
- Autophagy-dysregulated astrocytes: abnormal accumulation of Autophagosomes.
- Reduced mTOR and proteasome activitieswithin lysosomal dysfunction generate APDAs in an age-dependent manner.