AMPAR
links: Ionotropic Glutamate Receptor reference:
- AMPA receptors and their minions: auxiliary proteins in AMPA receptor trafficking probably everything I need to know. 4-13-2021
AMPA Receptor #
- Subunits are denoted as GluRx, GRIAx or GluAx. The receptor heterotetramer is usually two pairs; a dimer of dimers.
- AMPA is characterized by very fast activation/inactivation (milliseconds) kinetics and is mostly postsynaptic. Na+ flux to AMPARs induces depolarization.
-
Moderate AMPA receptor clustering on the nanoscale can efficiently potentiate synaptic current
- 50% increase in the synaptic AMPAR current could be provided by expanding the existing AMPAR pool at the expense of 100–200% new AMPARs added at the same packing density. Alternatively, reducing the inter-receptor distances by only 30–35% could achieve a similar level of current potentiation without any changes in the receptor numbers.
Calcium-permeable #
- The presence of GluR2 allows permability only to Na+, very rarely allowing Ca2+ influx. Without it, AMPAR apparently are also permeable to K+, Ca2+, and Zn2+. This is because Arginine residues (rather than glutamine) in GluR2 are positively charged. (I’m confused, is this ‘RNA editing’ of glu>arg endogenous??)
- When there’s excessive calcium and glutamate release, AMPAR becomes permeable to Ca2+.
- While Ca2+-permeable AMPA receptors are usually GluR2-lacking AMPA receptors, recent evidence suggests that Ca2+-permeable AMPA receptors containing unedited GluA2 do in fact occur in neurons and can contribute to excitotoxic cell loss.
-
GluA2-lacking, calcium-permeable AMPA receptors–inducers of plasticity?
- Cp-AMPARs are usually expressed transiently at an early stage of synaptic plasticity, but are then replaced with normal GluA2-containing receptors, indicating a role for Cp-AMPARs in induction, rather than the maintenance, of synaptic plasticity.
- Calcium-Permeable AMPA Receptors Mediate the Induction of the Protein Kinase A-Dependent Component of Long-Term Potentiation in the Hippocampus
-
Synapse Type-Dependent Expression of Calcium-Permeable AMPA Receptors
- Specifically expressed in cortical layer V.
- CP-AMPARs are synapse-specifically expressed at excitatory connections onto a subset of IN types in hippocampus and neocortex. For example, CP-AMPARs are found at connections from Pyramidal Neurons to basket cells (BCs), but not to Martinotti cells (MCs).
- Basket Cells are types of GABAergic Interneurons in the cortex, hippocampus, and cerebellum.
- Martinotti Cells are inhibitory and express Somatostatin and sometimes calbindin, but not parvalbumin.
- CP-AMPARs exhibit voltage-dependent channel block by Polyamines. Thus, intracellular spermine-dependent rectification is an oft-used proxy for Ca2+ permeability.
- GluR2-lacking CP-AMPARs desensitize faster.
- What is their use?
- Transient incorporation of native GluR2-lacking AMPA receptors during hippocampal long-term potentiation: Replaced by GluR2-containing AMPARs approximately 25 min after LTP induction.
Phosphorylation #
Naturally, all these (at least the serines) are on the C-terminus, which is the intracellular tail. GluR1’s is longer than GluR2’s.
-
Phosphorylation-Dependent Regulation of Ca2+-Permeable AMPA Receptors During Hippocampal Synaptic Plasticity (not finished - pretty juicy)
- It has been determined that ~15% of receptors are phosphorylated at S831 and S845 at rest
- CAMK II alone can be sufficient for LTP induction:
Long-term potentiation: peeling the onion
- Some CA1 synapses are postsynaptically silent, containing only NMDARs.
- Adjacent synapses are not affected during LTP.
- Following NMDAR activation CaMKII is translocated to the PSD by binding to the C-tail of the NR2B subunit and this interaction is important for LTP
- Mutated NR2B that prevents CAMK II translocation->binding still had 50% LTP of wild type.
- There was no requirement of the GluR1 C-tail for LTP. In fact, replacement with the GluR2 subunit showed normal LTP, as did an artificially expressed Kainate Receptor not normally found at these synapses. WTF?
- To add to the apparent complexity, the list of proteins proposed to be involved in LTP continues to grow (well over a hundred) leading some investigators to despair as to whether LTP is a tractable phenomenon: Can molecules explain long-term potentiation?
-
GluR1 Ser567: CAMK II
- Ca-Calmodulin does not enhance this, unlike with CAMK II at Ser831.
- Reduces synaptic localization. While LTP is associated with Ser831, this is for LTD.
-
GluR1 Ser816 & Ser818: Protein Kinase C.
- Increases channel conductance and in the cytoplasm, enhances 4.1N binding, which facilitates insertion (delivery and exocytosis (freeing the receptor itself from vesicle)).
- Facilitated by depalmitoylation of Cys-811.
- Increases channel conductance and in the cytoplasm, enhances 4.1N binding, which facilitates insertion (delivery and exocytosis (freeing the receptor itself from vesicle)).
-
GluR1 Ser743
-
GluR1 Ser831: Protein Kinase C or CAMK II.
- Increases channel conductance (independent of agonist efficacy) and opening probability/frequency, decreasing activation energy for the intrasubunit conformational change. Also mediates receptor trafficking.
-
GluR1 Ser845: Protein Kinase G II.
- Protein Kinase A downstream D1 Medium Spiny Neurons:
- Increases open probability, surface retention, and exocytosis.
-
GluR1 Cys811: Palmitoylation of GluR1 C811 residue modulated Protein Kinase C phosphorylation and GluR1 insertion R.
-
GluR1 Thr840: Protein Kinase C, S6K.
-
GluR2 Ser880: Protein Kinase C.
- Directly influences its C-terminus, its PDZ-binding domain
(R). Phosphorylation induces rapid internalization, since ABP/GRIP are now unable to bind. It can then interact with PICK1, which regulates its endocytosis to mediate LTD.
-
Glutamate receptor subunit 2 Serine 880 phosphorylation modulates synaptic transmission and mediates plasticity in CA1 pyramidal cells
- This study supposedly refutes the model that perhaps Ser880 phosphorylation disrupts intracellular retention. Idk; I think PICK1 might move GluR2 to and from the membrane.
-
Glutamate receptor subunit 2 Serine 880 phosphorylation modulates synaptic transmission and mediates plasticity in CA1 pyramidal cells
- Directly influences its C-terminus, its PDZ-binding domain
(R). Phosphorylation induces rapid internalization, since ABP/GRIP are now unable to bind. It can then interact with PICK1, which regulates its endocytosis to mediate LTD.
-
GluR2 Ser863
-
GluR2 Tyr876: Src family kinases.
-
Unlocking the secrets of the δ2 glutamate receptor
- Dephosphorylation allows S880 to be phosphorylated. GluD2 (δ2 glutamate receptor) maintains low phosphorylation via PTPMEG inhibiting Y876 phosphorylation.
-
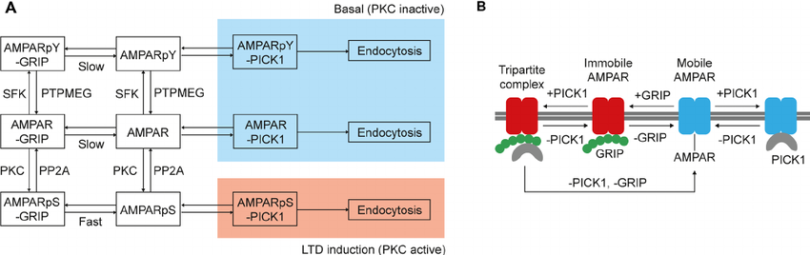 R
R - This PTPMEG is also bound to δ2 via its PSD-95 domain.
-
- Dephosphorylation allows S880 to be phosphorylated. GluD2 (δ2 glutamate receptor) maintains low phosphorylation via PTPMEG inhibiting Y876 phosphorylation.
-
Unlocking the secrets of the δ2 glutamate receptor
-
Dephosphorylated by PP1.
Insertion #
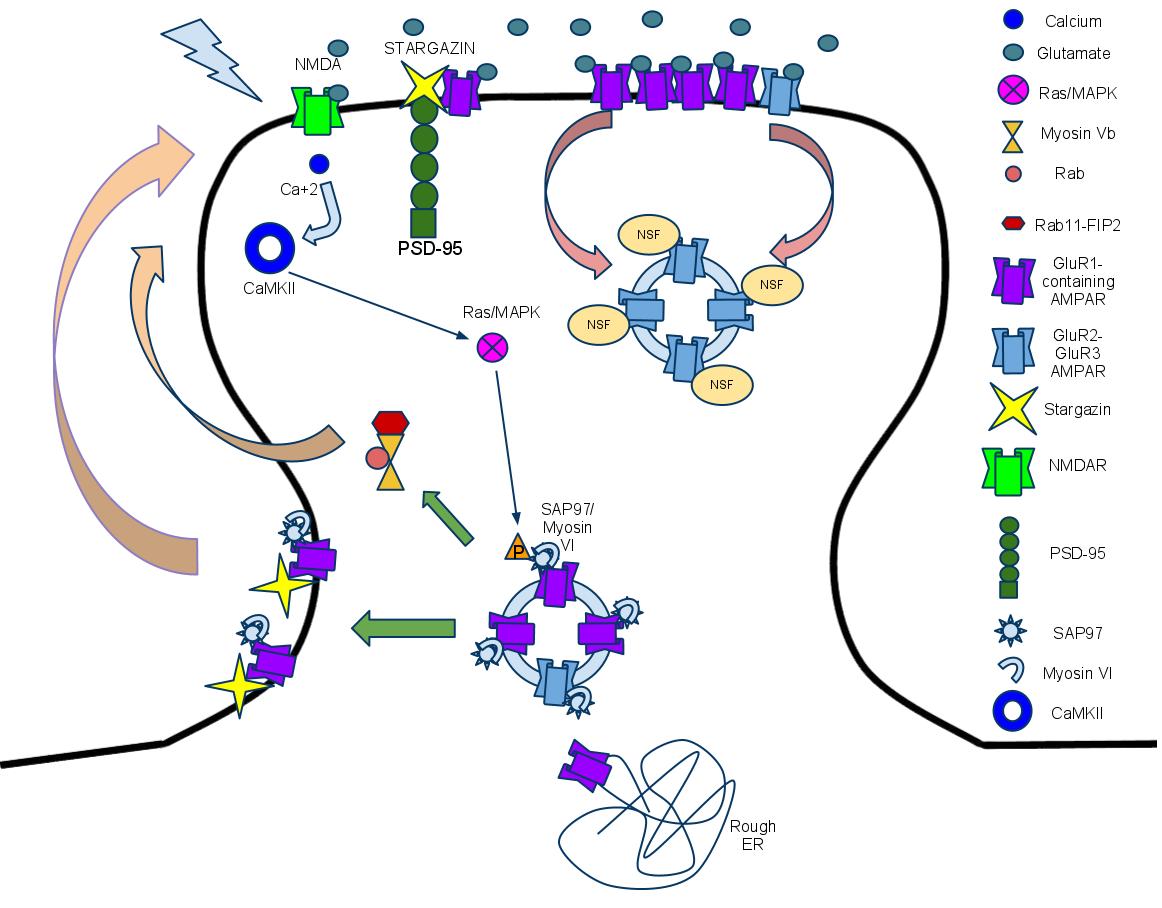
- I’m gonna guess that’s technically ERK1/2 phosphorylating myosin there. Good to know that ras/mapk is initiated by calmodulin.