P2X7
2022-04-30
P2RX7 (P2X purinoreceptor 7) #
-
-
-
ATP receptor; ionotropic. Found in microglia, macrophage, retina, endometrium.
- Requires higher ATP levels than other P2X, but response is potentiated by reducing concentration of divalent cations, as they block it.
- Some P2X receptors rapidly desensitize ATP, while ones like P2X2 remain open as long as ATP is bound.
- Technically, ADP and AMP are weak agonists.
- The ionotropic ATP receptor subunits P2X1–6 receptors play important roles in synaptic transmission, yet the P2X7 receptor has been reported as absent from neurons in the normal adult brain. R
- Requires higher ATP levels than other P2X, but response is potentiated by reducing concentration of divalent cations, as they block it.
-
Usually a dimer but can form heteromers with a few other P2X subunits.
-
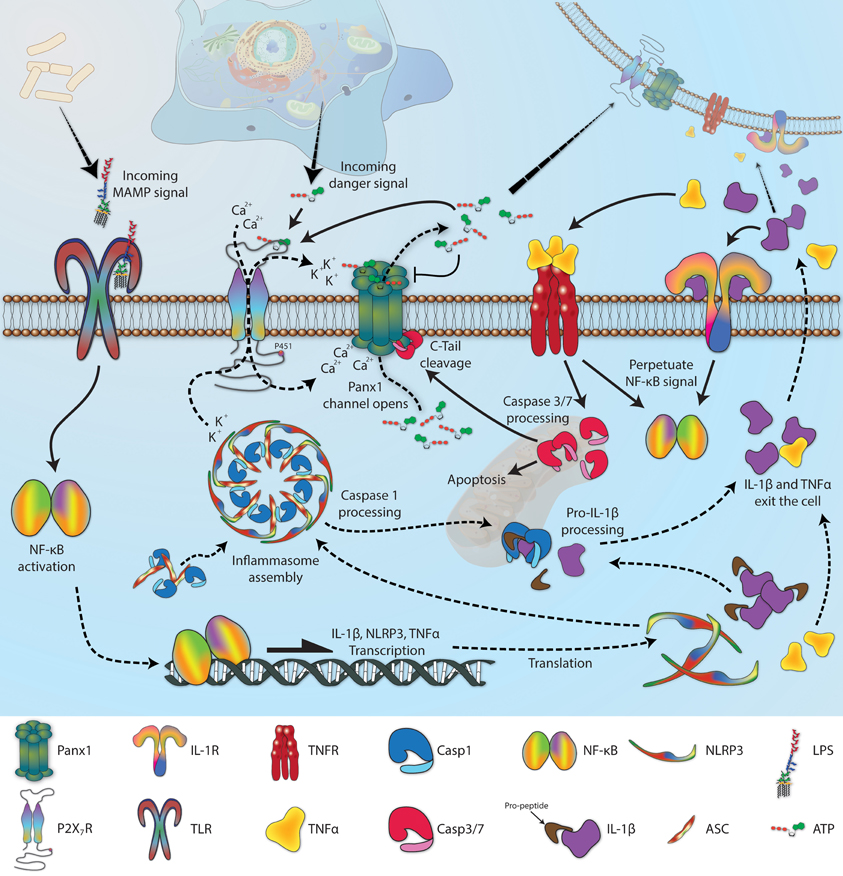
Activation leads to recruitment of Pannexin to form Panx1, a Ca2+-gated ATP export channel.
-
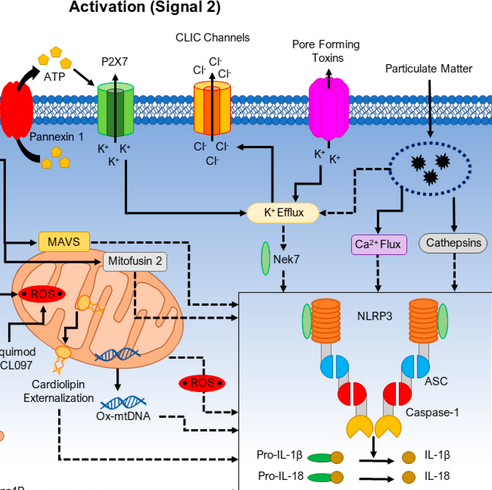
K+ efflux->PAF->NEK7 (NIMA-related kinase), binding to and activating NLRP3.
- Nek7 is an essential mediator of NLRP3 activation downstream of potassium efflux
- So how the hell does intracellular potassium of all things disinhibit this binding of Hek7?
-
A2A is the entry point from which cumulative sleep deprivation (ie aging/time passing) upregulates P2X7. P2X7 also upregulates A2A, so it’s a positive feedback loop. ATP release from extended P2X7 activation also activates P2X7, more positive feedback loop. This whole area is a ripe target.
-
- Activation of the purinergic P2X7 receptors is necessary and sufficient to convert maternal immune activation (MIA) to Autism-like features in male offspring mice.
-
Hyperactivation of P2X7 receptors as a culprit of COVID-19 neuropathology
-
P2X7 Receptors Amplify CNS Damage in Neurodegenerative Diseases #
Extracellular ATP #
-
The Elusive P2X7 Macropore
- Extracellular ATP causes reversible permeabilization of mammalian cell plasma membranes due to P2X7R-dependent formation of a large conductance pore (the ‘macropore’).
-
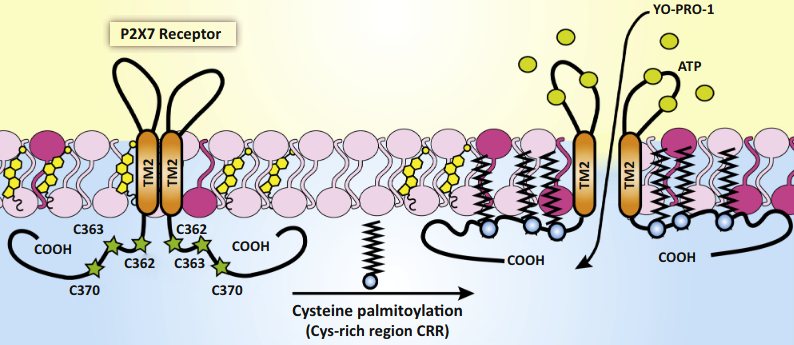
- Cholesterol in Lipid Rafts inhibits P2X7R-associated permeability increases. Palmitoylation opens it.
-
Regulation of P2X Purinergic Receptor Signaling by Cholesterol
- Defects in the C-terminal inhibited plasma expression of the receptor, probably leading to ubiquination.
- The activation of Acid Sphingomyelinase in microglia cells was shown to be necessary for P2X7 receptor-dependent microvesicle shedding and the release of IL-1β:
Acid sphingomyelinase activity triggers microparticle release from glial cells
- This causes a positive feedback mechanism as Ceramide and other sphingolipid is then able to displace cholesterol.
- This is not directyl related to obesity: The ATP-P2X7 signaling axis is dispensable for obesity-associated inflammasome activation in adipose tissue
- In macrophages, the synthesis of Leukotrienes through activation of PLA2 and mobilization of arachidonic acid is required for the P2X7-mediated reduction in the parasitic load of infected cells.
- Inhibition of connexin 43 hemichannel-mediated ATP release attenuates early inflammation during the foreign body response
Neurogenesis #
- As a ‘pattern recognition receptor’, its function is perhaps recognizing malformed/damaged neurons in response to extracellular ATP activity on Microglia. This is how cohesive connections are assessed during Neurogenesis. In highly neuroinflammatory states (like Alzheimer’s?) this process “feed forward”.
- https://en.wikipedia.org/wiki/Pattern_recognition_receptor also includes TLR, and some other things (CLR, RLR, NLR/NLRP).
-
The role of P2X7 receptors in a rodent PCP-induced schizophrenia model
- P2X7 knockout in mice promotes defects in cognition and social activity. IT Doubles surface expression of NR2A and NR2B.
- Impaired interleukin-1beta and c-Fos expression in the hippocampus is associated with a spatial memory deficit in P2X(7) receptor-deficient mice
- immature neurons fire much more quickly and are more disinhibited and have a much higher p2x7 receptor expression than should mature neurons. Increased p2x7 activity should disinhibit young neurons and that would be responsible for increased plasticity in young neurons but it also contributes to cell death