@Neuropharmacology of Sleep and Wakefulness (Watson et al. 2011)
2022-01-25 links: Sleep see: (728pgs.) Brain Control of Wakefulness and Sleep (2005)
@Neuropharmacology of Sleep and Wakefulness (Watson et al., 2011) #
-
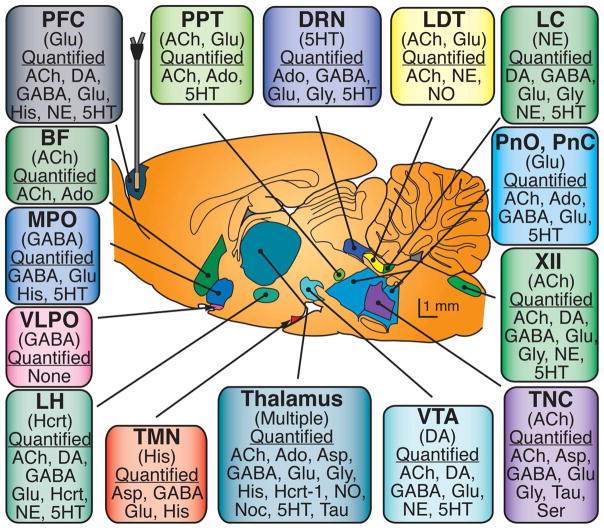 Fig 1 (Rat brain)
Fig 1 (Rat brain)
- The parenthesized neurotransmitters are the most notable for signaling to other brain regions, while the quantified NTs are those that have been measured to be relevant for arousal-state control within the region.
- Abbreviations: XII – hypoglossal nucleus; BF – basal forebrain; DRN – dorsal raphé nucleus; LC – locus coeruleus; LDT – laterodorsal tegmental nucleus; LH – lateral hypothalamus; MPO – medial preoptic area; PPT – pedunculopontine tegmental nucleus; PnC – pontine reticular formation, caudal part; PnO – pontine reticular formation, oral part; TMN – tuberomamillary nucleus; TNC – trigeminal nucleus complex; VLPO – ventrolateral preoptic area; VTA – ventral tegmental area; Hcrt – hypocretin (Orexin), Noc – nociceptin; Ser – serine
GABA #
-
Subtype differences (in location, and in effects?) are reviewed in: Role of GABAA receptors in the physiology and pharmacology of sleep
-
Although systemic administration of GABAmimetic drugs promotes sleep, sedation, or general anesthesia, enhancing GABAergic transmission within the Pontine Reticular Formation increases wakefulness. R, Evidence that wakefulness and REM sleep are controlled by a GABAergic pontine mechanism, (and many more Rs); GABA levels in it are greater during wakefulness than during REM.
- The posterior Hypothalamus as a whole
- This doesn’t follow for me at first - why would this not promote deep sleep or something? What would sleep EEGs look like under the influence of a lot of PPRF GABA?
-
As shown in the graph and explained above and by the fact it’s obvious, enhanced GABAeric inhibiton in (certain?) wakefulness-promoting brain regions causes an increase in sleep, including the Dorsal Raphe Nucleus, the Tuberomamillary Nucleus, medial preoptic area, and ventrolateral periaqueductal gray.
Acetylcholine #
Cholinergics aren’t really part of the standard pharmacological armamentarium of sleep disorder medicine.
- It’s M2 AChR that plays a key role in REM generation, (specifically?) signaling originating from the laterodorsal tegmental and pedunculopontine tegmental nuclei, and the Basal Forebrain.
- One population of LDT/PPT dicharges during wakefulness and REM, and one during only wakefulness.
- There are LDT/PPT terminals and muscarinic receptors in the Pontine Reticular Formation.
- Intravenous eszopiclone, a shitty sleep medication and GABA-A positive allosteric modulator, prevented REM, increased Delta waves power, and dcreased Acetylcholine release in the PRF.
- Cholinergic neurons originating in the Basal Forebrain project throughout the entire cerebral cortex, with acetylcholine release therein being highest during REM, lower during wakefulness, and lowest during NREM.
Adenosine #
- ATP levels increase during sleep in areas of the brain that are most active during wakefulness. (Sleep and brain energy levels: ATP changes during sleep)
- Prolonged wakefulness increases adenosine levels selectively in the basal forebrain/cortex and increases Adenosine A1 binding. The basal forebrain does not contain A2A
- [Opposite changes in adenosine A1 and A2A receptor mRNA in the rat following sleep deprivation]
- Inactivation of A1 decreases Delta waves power and NREM sleep time.
Serotonin #
-
The monoamine-containing neurons, excluding dopamine, discharge at their fastest rates during wakefulness, slow during NREM, and cease prior to and during REM.
- Serotonin release in the Dorsal Raphe Nucleus and preoptic area follows this pattern; it’s highest during wakefulness.
-
Agonists of 5-HT1A, 5-HT1B, 5-HT2A, and 5-HT2C decrease REM specifically, and then long with 5-HT3 cause an increase in wakefulness and decrease in sleep. (The roles of dopamine and serotonin, and of their receptors, in regulating sleep and waking)
- It follows that mice lacking genes for 1A or 1B show an increase in REM.
- Local administration of 5-HT1A agonist to the Dorsal Raphe Nucleus increases wakefulness in rats but increases REM in Katzen!
- Microinjection of 2A/2C agonist into rat dorsal rapeh nucleus decreases REM with no signigifcant effect on wakefulness.
- Antagonism of 5-HT2A 5-HT6 decreases wakefulness, increases NREM, and has not effect on REM. These data are consistent with the view that serotonin is wakefulness-promoting
Noradrenaline #
I mean, no duh it’s wakefulness/arousal-promoting, but it’s brain region specific.
- Noradrenergic cells of the Locus Coeruleus inhibit REM, promote wakefulness, and project to a variety of other arousal-regulating brain regions including the Prefrontal Cortex.
- Bilateral microinection of α1-agonist, α2-agonist, or β-antagonist into the pedunculopontine tegmental nucleus increases REM with little/no effect on NREM or wakefulness.
Histamine #
- Projections from the Tuberomamillary Nucleus are wakefulness-promoting. They turn off during sleep.
Dopamine #
The involvement of dopamine in the modulation of sleep and waking
- Arousal-regulating cell bodies reside in the Ventral Tegmental Area and the Pars Compacta, but they do not change firing rates as a function of states sleep/wakefulness.
- They project to the Dorsal Raphe Nucleus, Basal Forebrain, Locus Coeruleus, Thalamus, and Laterodorsal Tegmental Nucleus.
- There are also DA neurons in the ventrolateral periaqueductal gray R that are active during wakefulness with recipocal connections with sleep-regulating brain areas.
- DAT knockout increased wakefulness and decreased NREM.
- D1 agonism/antagonism increases/decreases wakefulness, and intracerebroventricular D1 or D2 agonists to rats increases wakefulness.
Glutamate #
Glutamate levels in some areas of the rat cortex increase during wakefulness/REM, decreasing during REM.
- Glutamate modulates traits/states of arousal in the LDT/PPT, Pontine Reticular Formation, and medial portions of the medllary Reticular Formation.
Orexin #
Leptin and Ghrelin #
Opioids #
- They disrupt sleep (which increases pain lol). A single intravenous infusion of morphine in healthy volunteers increases stage 2 NREM, decreases stage 3 and 4 NREM and REM.
- The REM disruption is mediated at least in part by decreasing acetylcholine in the Pontine Reticular Formation. Opioids decrease Adenosine levels in the basal forebrain and in the Pontine Reticular Formation.