Muscle
links: reference:
- Andy Galpin 1/3 https://www.youtube.com/watch?v=MyKrc-fheBw
- https://www.physio-pedia.com/Golgi_Tendon_Organ 6-3-2021
Muscle #
-
Phosphoproteomics of three exercise modalities identifies canonical signaling and C18ORF25 as an AMPK substrate regulating skeletal muscle function
- C180ORF25… KO decreases exercise capacity, muscle function, and PKA signaling.
- Phosphorylated on S67 by AMPK.
- Supposedly only contributes to ~20% of the TDEE, at ~15cal/kg per day. At rest, muscles use fat for ~90% of their energy. They use about 144g of glucose a day. Along with 100-200g with the liver and 120-150g for the brain, you can consume 400-500g carbs before any lipogenesis, and that’s assuming you’re in a big caloric surplus.
Hypertrophy #
- Why exercise builds muscles: titin mechanosensing controls skeletal muscle growth under load
- It’s not really known, and it’s mostly a theory, as for how microtrauma and whatnot actually leads to hypertrophy Resistance training‐induced changes in integrated myofibrillar protein synthesis are related to hypertrophy only after attenuation of muscle damage
- Growth Hormone and IGF-1 induce muscle hypertrophy and stimulate activity on endocrine tissues. They also increase the sensitivity of target cells to Thyroid hormones. R
- Apparently skeletal muscle cells contain multiple nuclei to reach a larger area, considering they’re very large cells. And the more nuclei there are, the larger the potential size of the cell. Myonuclei accumulation is key to ‘muscle memory’.
-
Myonuclei acquired by overload exercise precede hypertrophy and are not lost on detraining
- Even the myonuclei acumulated from anabolic steroid use remain after cessation.
- The nuclei are accumulated from satellite cells:
-
Adult stem cells at work: regenerating skeletal muscle
-
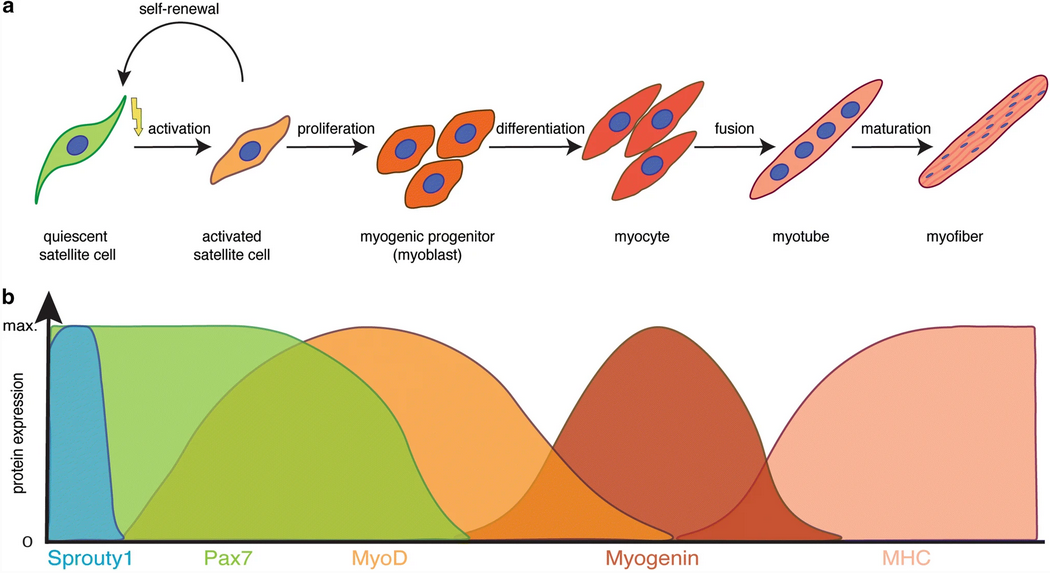
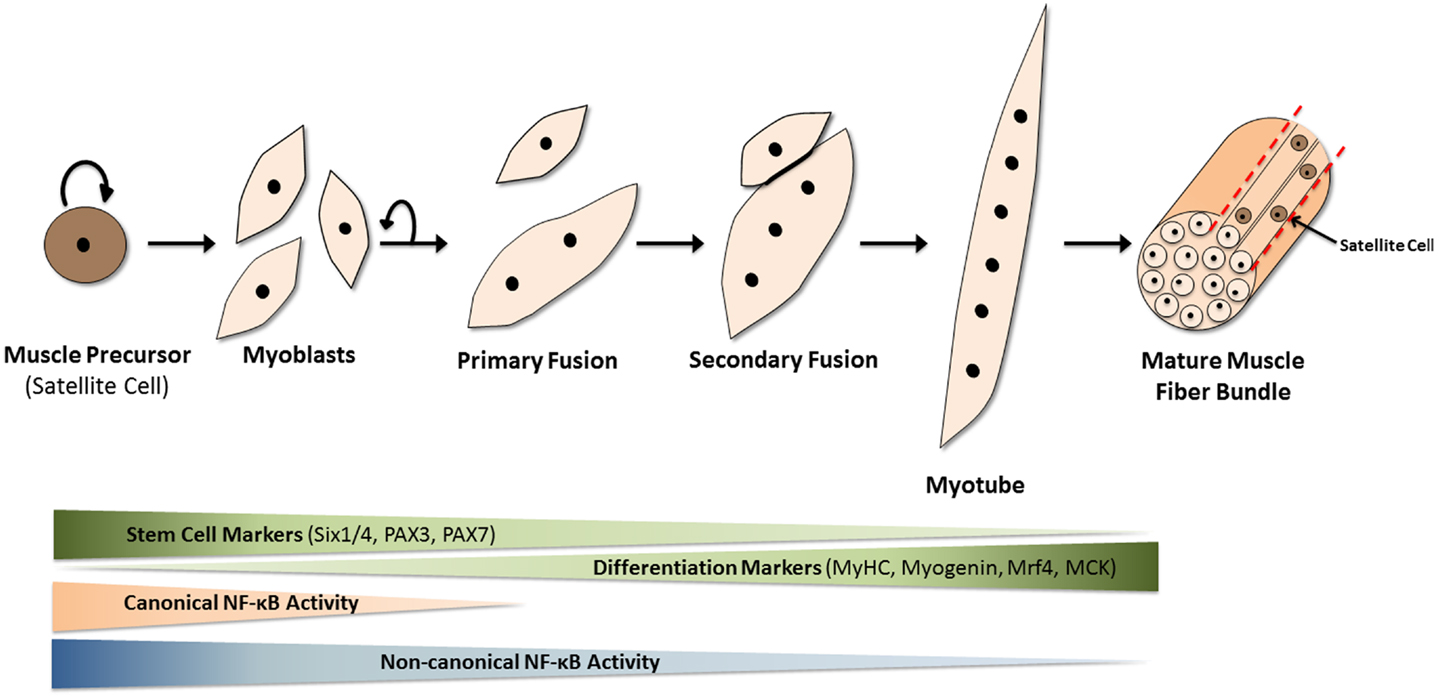
-
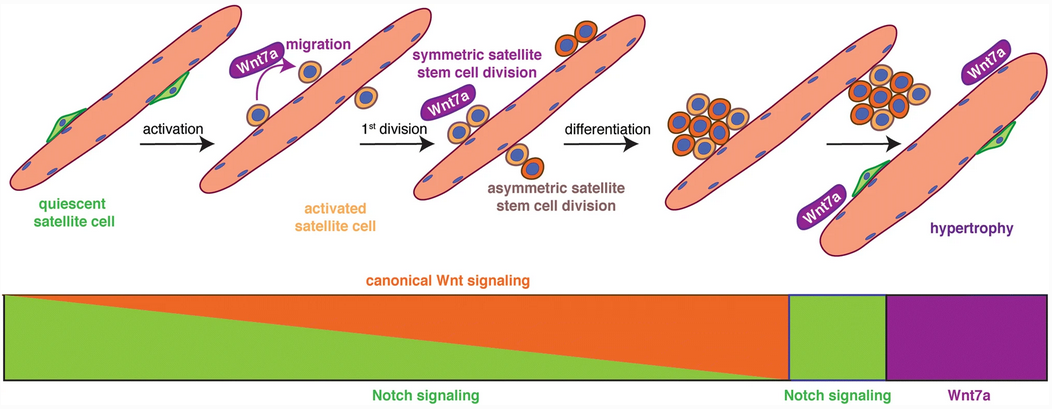
- So Notch promotes proliferative signaling. It’s part of Neurogenesis as well.
- Note WNT7a
-
-
Adult stem cells at work: regenerating skeletal muscle
-
Myonuclei acquired by overload exercise precede hypertrophy and are not lost on detraining
Anatomy #
-
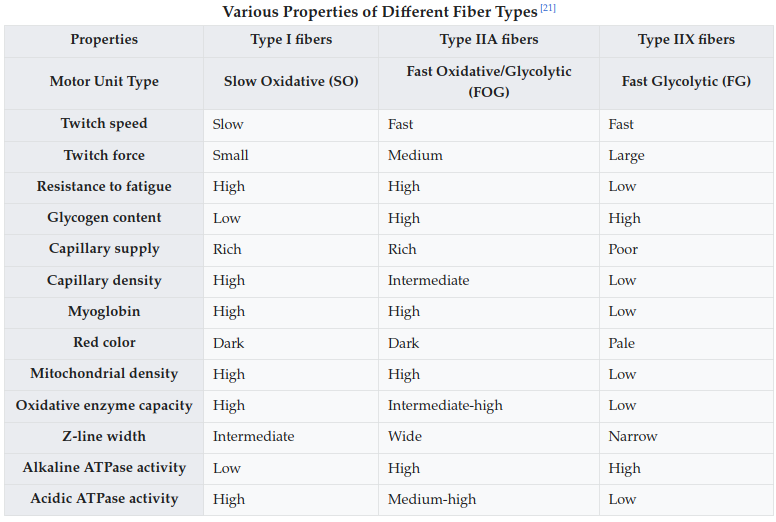
- The alkaline vs acidic ATPase one is interesting. Unless I’m mistaken this might imply Type I is optimized for lactate buildup.
- I mean on the contrary, 2b>2a>1 for anaerobic capacity. Also 1>2a>2b for triglyceride stores.
- Little association between the amount of reps you can get as % of your 1RM and fiber type. It is a function of capillarization though.
- The alkaline vs acidic ATPase one is interesting. Unless I’m mistaken this might imply Type I is optimized for lactate buildup.
- Muscles are excitable via a membrane potential, akin to neurons, by connected Motor Neurons releasing Acetylcholine. This is what causes contraction, which begins at the myofibrils.
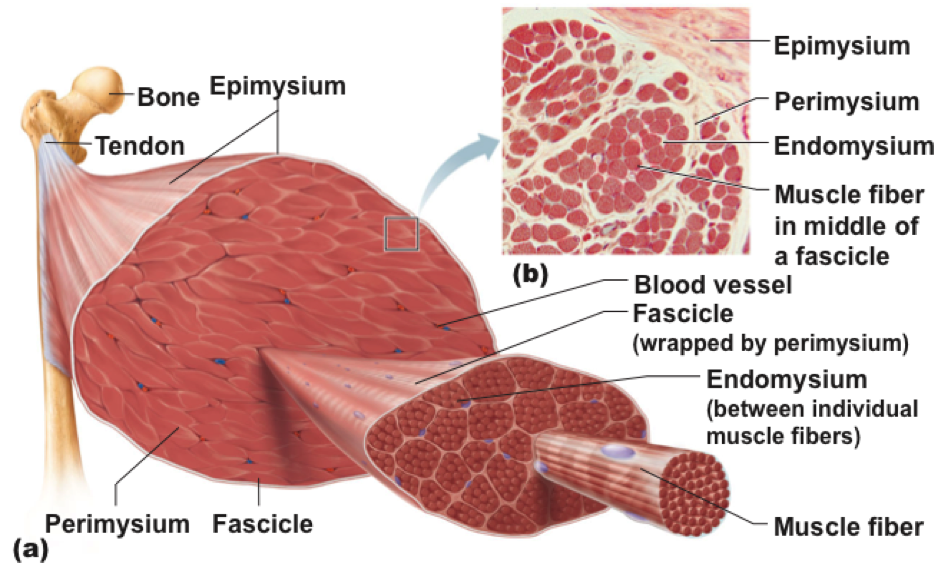
- This pulls on all the connective tissue sheets (-mysium), which pull on the tendons, ultimately pulling a bone. There will always be a bone that moves, and one that doesn’t. The former is the insertion, the latter the origin. An example is a masseter, with the zygos and mandible.
- The adapative response to stress/tension/damage is the synthesis of Myosin, which leads to hypertrophy of the muscle head by:
- Myofibers can grow in length. Muscle fibers only go 1/2 or 1/4 or so of the length of a muscle, but they can extend further. This would seem to lead to additional sarcomeres. It’s not the typical response, though, and it’s not known for sure if this occurs at the fiber-level or just the fascicle.
- Fascicles can change in pennation angle, from 7-14% or so. This makes the muscle “taller”, so to say.
- New fibers are not synthesized under normal circumstances - that is hyperplasia, not hypertrophy.
- Fibers can possibly split like hairs/twizzlers to regrow in size later. Hypertrophy most likely occurs due to the radial growth of myofibers, either by creating thicker myosin within the sarcomere, hypertrophy of the sarcoplasm (thus non-contractile units) from water and such.
- This is very uncommon and probably limited to steroid users.
- Myofibers can grow in length. Muscle fibers only go 1/2 or 1/4 or so of the length of a muscle, but they can extend further. This would seem to lead to additional sarcomeres. It’s not the typical response, though, and it’s not known for sure if this occurs at the fiber-level or just the fascicle.
σάρξ = “flesh”.
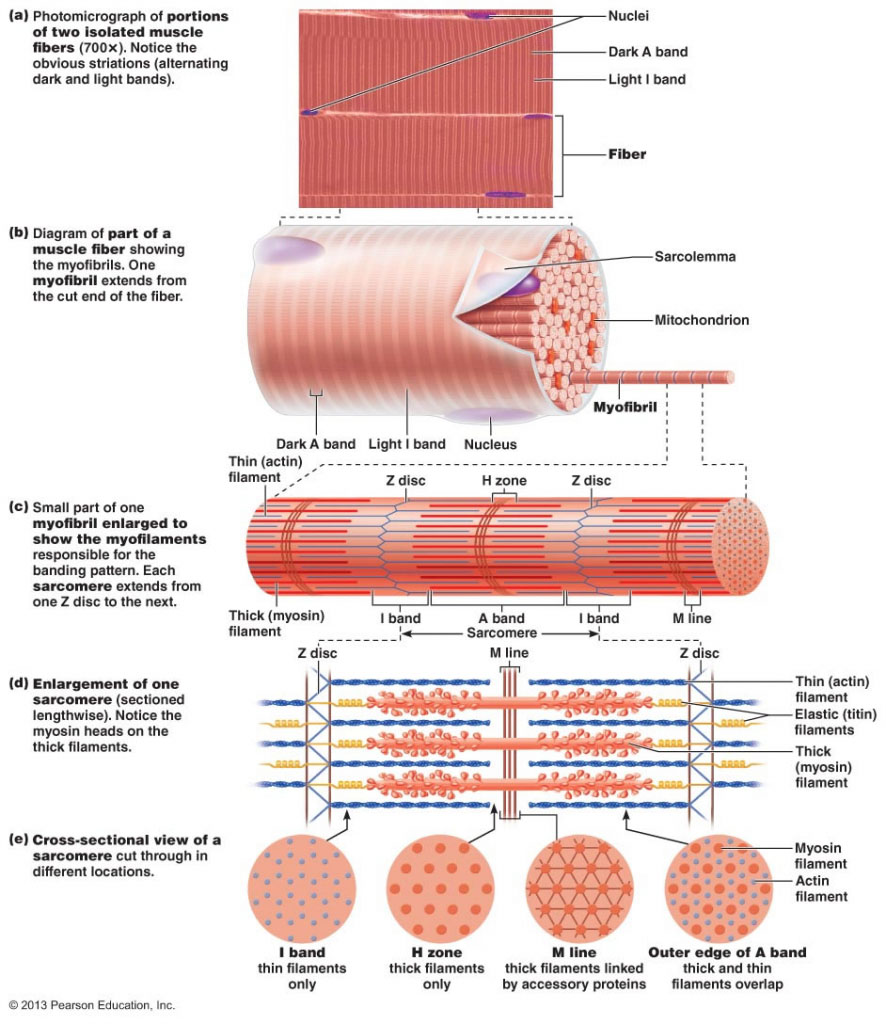
- A muscle fiber/myofiber is a single cell, one of the largest in all of biology - they can be seen with the naked eye. Most fast-twitch muscle fibers are larger than slow-twitch.
- Muscle fibers are ~75% water, 15% myofibrillar proteins, 5% sarcoplasmic “proteins?”.
- 50% of the protein is ~50% myosin, ~20% actin, ~10% titin, ~5% nebulin, troponin, tropomy, 5% sarcoplasmic reticulum, Mitochondrial, T-tubules, etc.
- Muscle fibers are ~75% water, 15% myofibrillar proteins, 5% sarcoplasmic “proteins?”.
- Sarcolemma = Plasma membrane
- Sarcoplasm = Cytoplasm
- Sarcoplasmic reticulum = Endoplasmic reticulum; produces calcium.
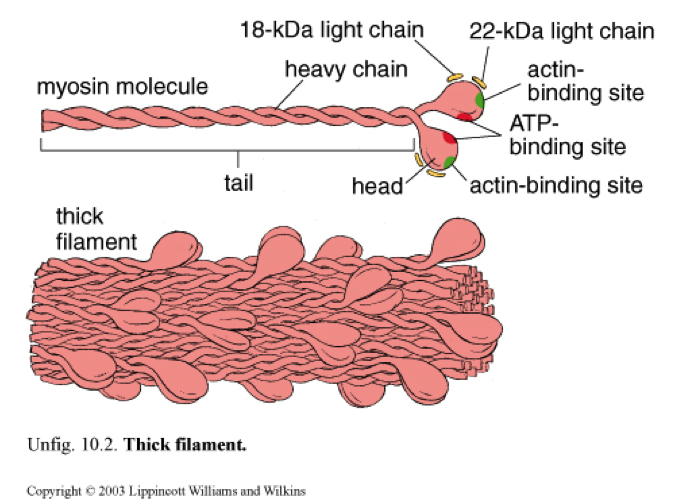
Sarcomere #
Going down deeper than the fiber is the myofibril - fibers can contain hundreds or thousands.
Within myofibril are sarcomeres.
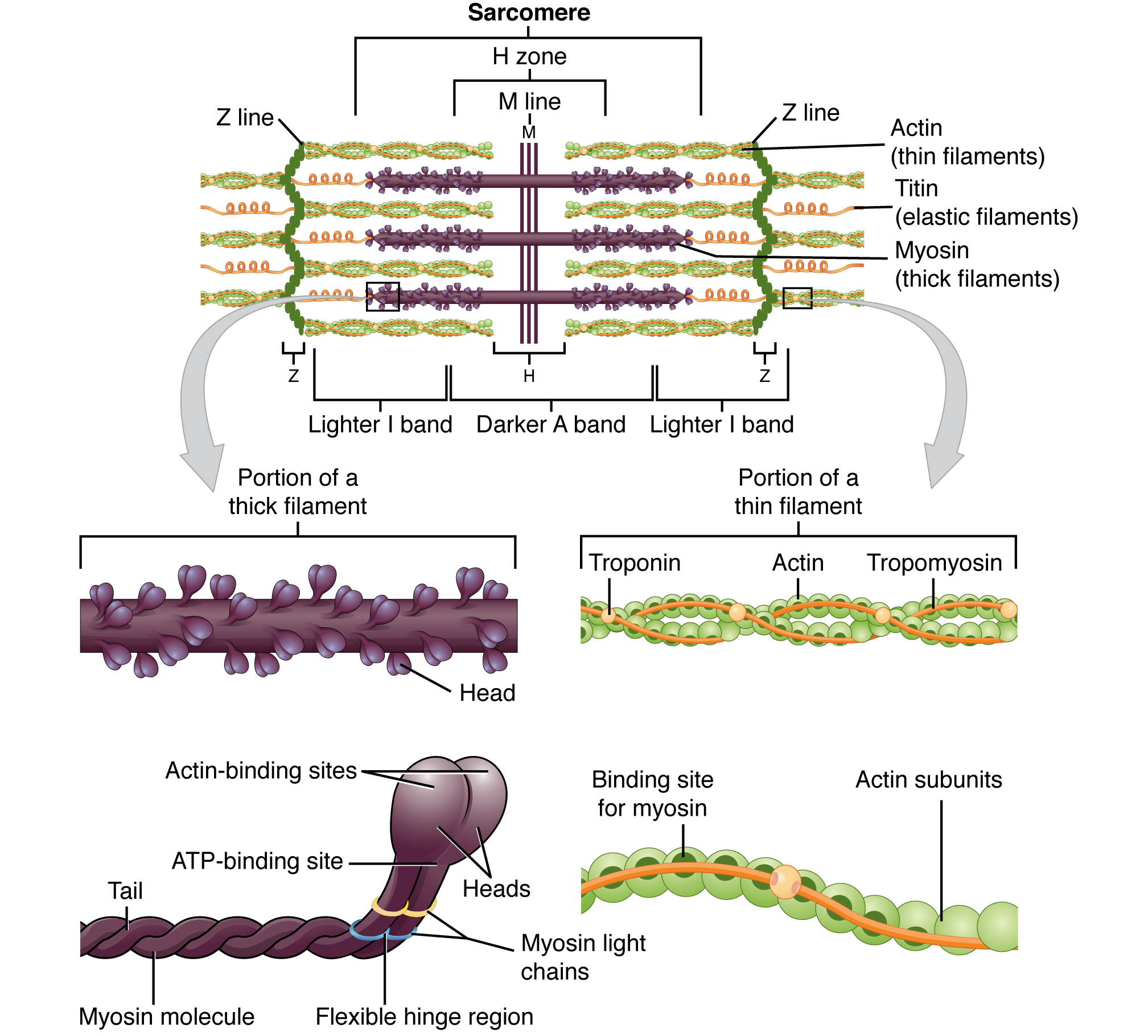
-
From Z-disc to Z-disc is one structural/functional unit of a sarcomere, as seen in the figure.
- Z-discs are made of α-actinin.
-
The A-band covers the distance of the thick filaments that run down the sarcomere. Between them and the Z-discs (and extending into adjacent sarcomeres) are the I-bands: titin, which bind myosin to the Z-discs.
-
In the middle of the sarcomere is the M-line, connecting myosin to accessory proteins. Titin also binds the two together upon intersection.
- The M-line is made up of myomesin, creatine kinase, and c-proteins.
-
The thin (Actin) filament runs parallel with everything else.
- It’s anchored to the Z-disc by nebulin, a structural-supportive protein.
- Dystrophin links the thin filaments to the sarcolemma.
- The base of the head of Myosin binds to active sites (holes) inside F-actin (filamentous; as opposed to G-actin, globular, the unpolymerized, individual form) (F-actin gives rise to the helix-like shape of the thin filament due to it being a polymer)
- Tropomyosin, a cord-like protein, blocks the active sites when the muscle is at rest, preventing myosin from binding.
- Troponin, another protein of course inside the thin filament, binds to actin, tropomyosin, and calcium ions, the specific sites being known as Troponin-I (inhibitory), Troponin-T, and Troponin-C respectively. When calcium binds to Tn-C, it alters the shape of Tn-T, pulling on tropomyosin, opening up active sites for myosin to contract the muscle.