IRS-1
2022-05-16: reference:
Insulin Receptor Substrate 1 (IRS1) #
A binding site and signaling protein of the insulin receptor. It is the means by which the insulin receptor goes to active PI3K.
- I believe it is directyl inhibited by free fatty acids.
Phosphorylation #
- Insulin receptor -> autophosphorylation -> intrinsic kinase -> IRS-1 tyrosine phosphorylation, where these tyrosine residues serve as docking sites for SH2 (Src homology 2) domain-containing proteins, namely PI3K.
- S6K and mTORC1 phosphorylates (inhibits) IRS-1 as part of a negative feedback loop. As does JNK, ERK, PKC.
- The negative sites lead to downregulating IRS-1 protein levels via ubiquinaton, inhibiting the interaction between activated insulin receptor and the PTB (phosphotyrosine) domain, or by messing with p85 (PI3K regulatory subunit)
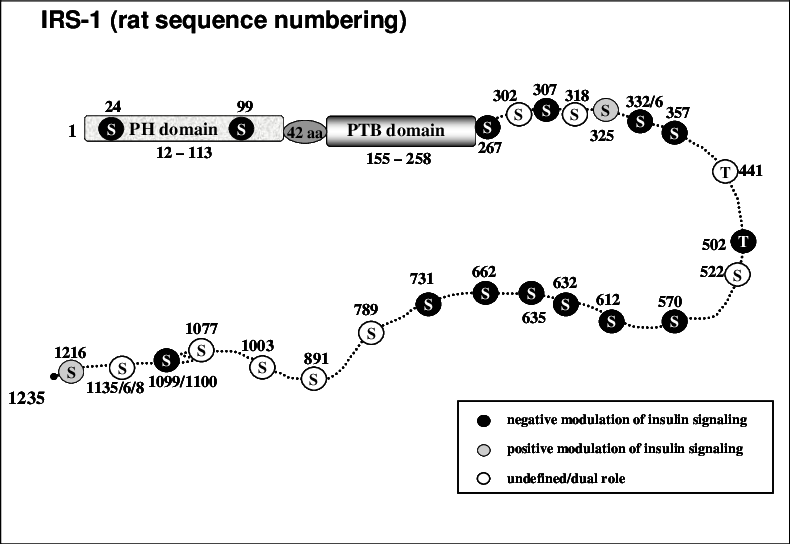
- The negative sites lead to downregulating IRS-1 protein levels via ubiquinaton, inhibiting the interaction between activated insulin receptor and the PTB (phosphotyrosine) domain, or by messing with p85 (PI3K regulatory subunit)
- Ser307 (murine Ser302): its main effect is that it prevents tyrosine phosphorylation.
- Mediated by S6K, i.e. from the PI3K-AKT-mTOR pathway, which is why mTOR inhibits this Ser307 phosphorylation.
-
Phosphorylation of IRS1 at serine 307 in response to insulin in human adipocytes is not likely to be catalyzed by p70 ribosomal S6 kinase
- S6K1 does not phosphorylate IRS1 at serine 307 in response to insulin in intact human primary adipocytes.
- We first analyzed the time-course for phosphorylation of IRS1 at Ser307 in response to a maximal effective concentration of insulin, and compared it with that of the phosphorylation of the ribosomal protein S6 (S6) at serine 235/236, which is a bona fide physiological substrate of S6K1.
- Phosphorylation of IRS307 reached steady-state after 5 mins, while S6 phosphorylation had a 5 minute lag, then reached steady-state after 30 minutes.
- Cells were transfected with a dominant negative construct of S6K1 (S6K1-DN) with the mTORC1 phosphorylation site at threonine 389 substituted with alanine, which precludes activation of the S6K1-DN by insulin.
-
Phosphorylation of IRS1 at serine 307 in response to insulin in human adipocytes is not likely to be catalyzed by p70 ribosomal S6 kinase
- The c-Jun NH(2)-terminal kinase promotes insulin resistance during association with insulin receptor substrate-1 and phosphorylation of Ser(307)
- On another note: Salicylic acid reverses phorbol 12-myristate-13-acetate (PMA)- and tumor necrosis factor alpha (TNFalpha)-induced insulin receptor substrate 1 (IRS1) serine 307 phosphorylation and insulin resistance in human embryonic kidney 293 (HEK293) cells
- Mediated by S6K, i.e. from the PI3K-AKT-mTOR pathway, which is why mTOR inhibits this Ser307 phosphorylation.
- Ser323: phosphorylation increasd in type 2 Diabetes
- Ser348 & Thr446 & Ser1100 & Ser1142: Insulin suppresses phosphorylation
- Ser527 & 531: Insulin increases its phosphorylation
- Ser636/639: negative. Decreased by Ser629 phosphorylation