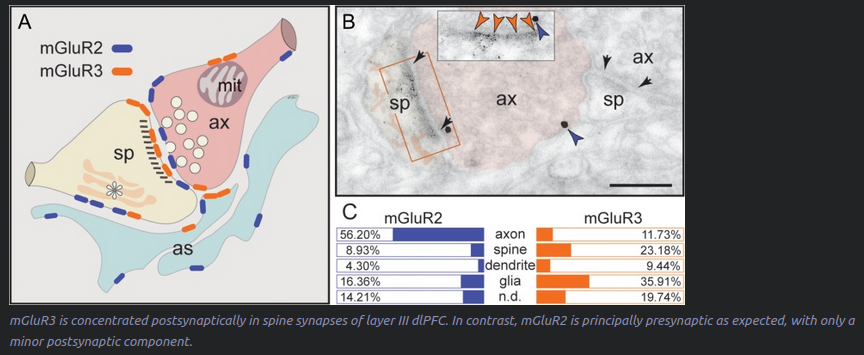
mGluR3
2023-03-27:
mGluR3 #
-
mGlu3 – New hope for pharmacotherapy of schizophrenia shortish
-
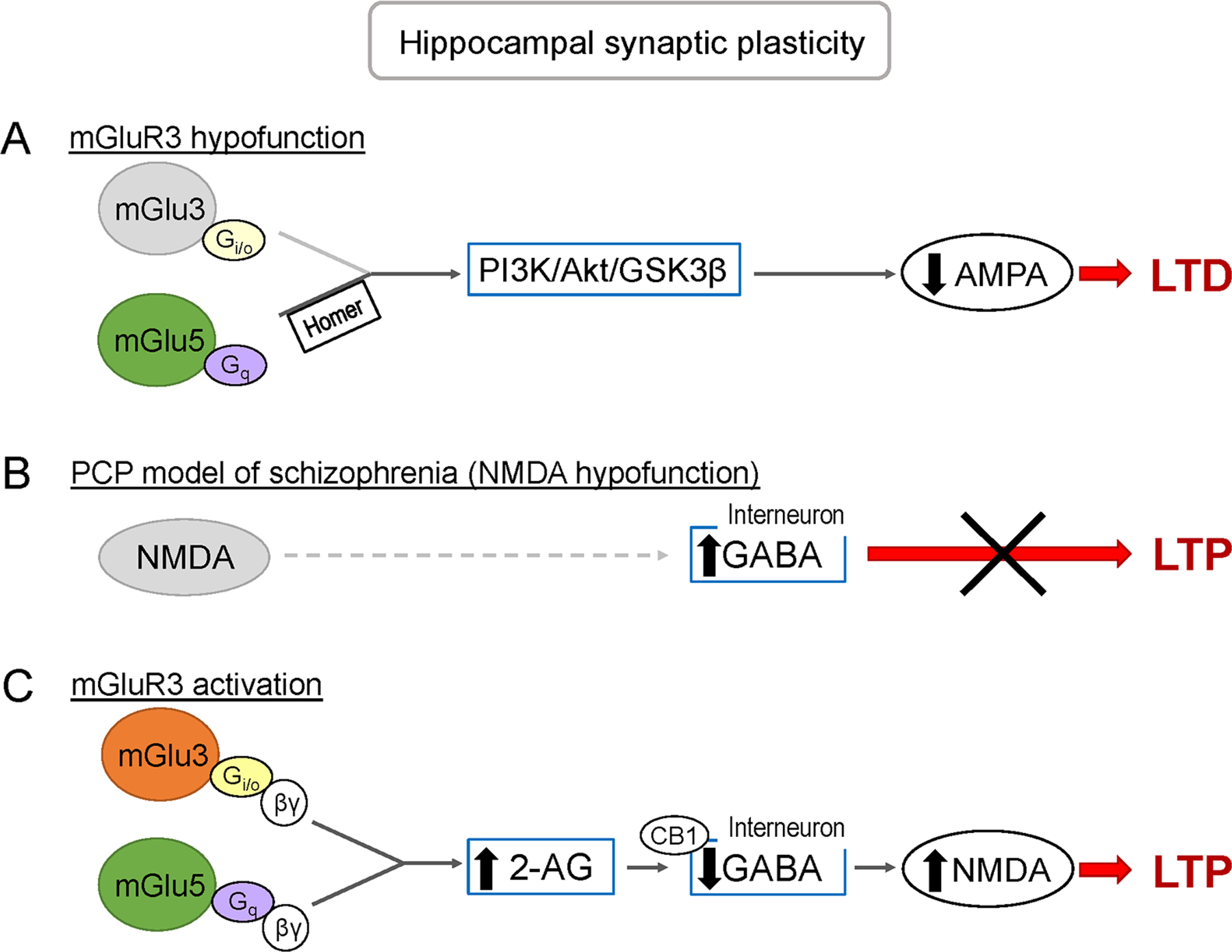
- Activation of mGlu3 switches mGlu5-mediated plasticity towards the generation of LTP by promoting 2-Arachidonylglycerol-mediated inhibition of GABAergic interneurons (disinhibition)
-
- [The Evolutionary Expansion of mGluR3-NAAG-GCPII Signaling: Relevance to Human Intelligence and Cognitive Disorders (2020)] #Read
- The previous view on mGluR3 in research emphasized their action on presynaptic glutamate terminals, doing Gαi stuff. Lame!
- mGluR3 are localized on astrocytes where they enhance glutamate uptake via EAAT
-
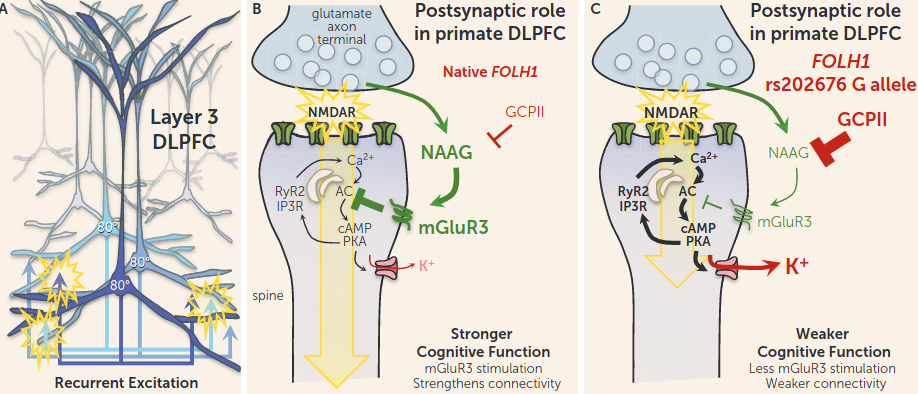
- mGluR3 (located on dendritic spines) activation is key for evolutionarily novel, layer III dlPFC microcircuits that subserve Working Memory. Keeping AC→cAMP/PKA→HCN low to keep signal propagation high. recurrent excitation keeps information(/engrams?) “in mind”.
- I.e., mGluR3 facilitates feed-forward signaling. With low GCP-II activity, neuraonal firing rate is increased.
- But like, is this not crazy though? How many nootropics increase cAMP/Ca2+?? I wonder if the localization of mGluR3 is what makes all the difference. Far away enough to not interfere with plasticity?
- inhibition of cAMP signaling closes K+ channels, strengthens connectivity, and enhances the persistent neuronal firing needed for working memory (5, 7).
- I wonder if α2A agonism, like from guanfacine of course, facilitates this? (Holy shit this is the same author as that one paper on guanfacine! Same figures and everything
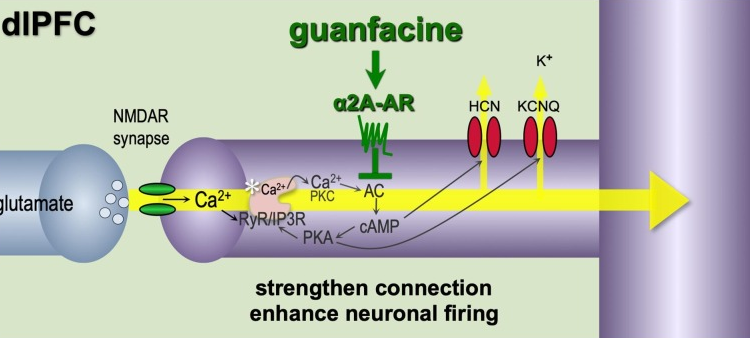 )
)
- human subjects with the FOLH1 variant would have less NAAG stimulation of mGluR3, greater opening of K+ channels, and weaker DLPFC network connectivity needed for working memory and abstract reasoning (Figure 2C) and thus would have to recruit greater amounts of DLPFC to perform a cognitive task (i.e., inefficient action of the DLPFC as seen in the Zink et al. study). Because GCPII expression is also increased by inflammation, we can speculate that similar insults to cortical connectivity and cognitive function may occur during an infection, perhaps helping to explain why we often have “brain fog” during illness
- Advancing age is associated with loss of mGluR3 and PDE4, as well as high levels of cAMP-HCN in dlPFC delay cells:
- Stress weakens prefrontal networks: molecular insults to higher cognition (Arnsten 2015) same old same old as Arnsten’s other dlPFC studies, but then it goes into actin/microtubules.
- The closing remark: the role of GCPII-NAAG-mGluR3 signaling expands with cortical evolution to regulate the circuits underlying human intelligence
-
mGluR2 versus mGluR3 Metabotropic Glutamate Receptors in Primate Dorsolateral Prefrontal Cortex: Postsynaptic mGluR3 Strengthen Working Memory Networks
-
-
The Metabotropic Glutamate Receptor mGluR3 is Critically Required for Hippocampal Long-term Depression and Modulates Long-term Potentiation in the Dentate Gyrus of Freely Moving Rats
- agonist activation of mGluR3 modulates LTD at a presynaptic locus. NAAG impaired the expression of LTP.
- NAAG effects on LTP were blocked by EGLU, a selective group II mGluR antagonist