G-protein
2022-02-15 links: reference:
G-protein (Guanine nucleotide-binding proteins) (GTP-binding protein) #
_
Heterotrimeric (“Large”) #
Activated by GPCR: 18 different Gα, 5 Gβ, and 12 Gγ
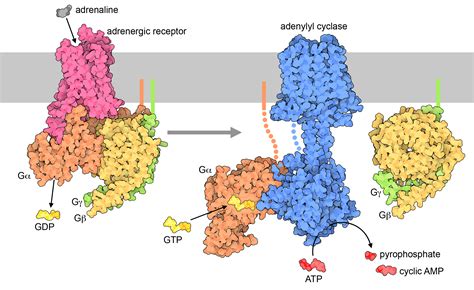
- A heterotrimeric G protein is composed of an α protein and a Gβγ complex.
- When inactive, the Gα is bound to GDP. When stimulated via ligand binding, the GPCR directly undergoes conformational change, and functions as a GEF domain, which fcilitates GDP’s dissociation from Gα, which otherwise happens very slowly. The Gα then binds to GTP and dissociates from the βγ, and goes to bind to an enzyme, i.e. AC.
- (I’m a bit confused what a GEF actually is. Wikipedia says that the binding of GTP to a GTPase releases the GEF, which goes to activate a new GTPase. But I thought they were intrinsic protein domains of the α subunits? Do they really bind and unbind?)
- The binding of one ligand to a GPCR activates >1 G-protein, thus multiple of the proceeding complexes.
- Then, dephosphorylation/hydrolysis via an intrinsic GTPase in the α subunit results in closure of the channel, and a reassociation to GDP and βγ. This usually takes about a few seconds.
- GAPs (GTPase-activating/accelerating proteins) can accelerate this process, further limiting the lifetime of Gα-GTP. Some GPCRs are almost entirely dependent on them.
- The remaining cascade still must terminate by other means: cAMP is deactivated by PDE, and the action of PKA (when its catalyst cAMP runs out, it does) is deactivated by Protein Phosphatases, cleaving the phosphate from whatever proteins.
- The βγ doesn’t stay there; it can go on to do its own signalling cascades. This is the case for each alpha subunit I believe.
- Gβγ from Gi tends to activate K+ channels.
-
G PROTEIN βγ SUBUNITS Annual Review of Pharmacology and Toxicology
- Gβγ is now known to directly regulate as many different protein targets as the Gα subunit!
- Gαs stimulates Adenylyl Cyclase.
- Gαi inhibits Adenylyl Cyclase of course, but activates GIRK/ir Potassium Channels.
- Gαo inhibits VGCCs (N- and P/Q-type).
- Gαq activates Phospholipase C, which cleaves Phosphatidylinositol 4,5-biphosphate (PIP2) into Inositol Triphosphate + Diacylglycerol.
- IP3 is soluble. It binds to IP3 receptors (ligand-gated Ca2+ channels) on the membrane of the Smooth Endoplasmic Reticulum, eliciting Ca2+ release from its internal stores into the cytosol. It then does stuff like binds to Calmodulin, which activates CAMK, which can activate CAMKK. Surely CAM-KKK is next. Too racist.
- Diacylglycerol remains in the membrane and recruits Protein Kinase C from the cytoplasm. Since its α/β/γ isoforms need Ca2+, the two second-order effectors of PLC do work in synchrony in this simple sense.
- Decreased membrane PIP2 levels results in reduced activity of PIP2-sensitive channels like KCNQ.
- Gα12/13 activates Rho small GTPases (RhoA, Cdc42, Rac1) via
- The Ras superfamily is the easiest example of small GTPases.
Small GTPases (small G-proteins) #
Homologous to the α subunit of heterotrimeric GTPases, so they independently hydrolyze GTP to GDP.
- Ras GTPases (‘Ras’): there’s maybe 30 of ’em.