Protein Folding
2022-04-08: reference:
Protein Folding #
-
One considers the incomplete protein an unfolded polypeptide/random coil. Still in the ribosome, this linear chain folds into the native state (tertiary structure) after its bonds to the other units is complete.
- Random coils are also found in complete proteins where they do not form a (consistent/recognized secondary?) structure.
-
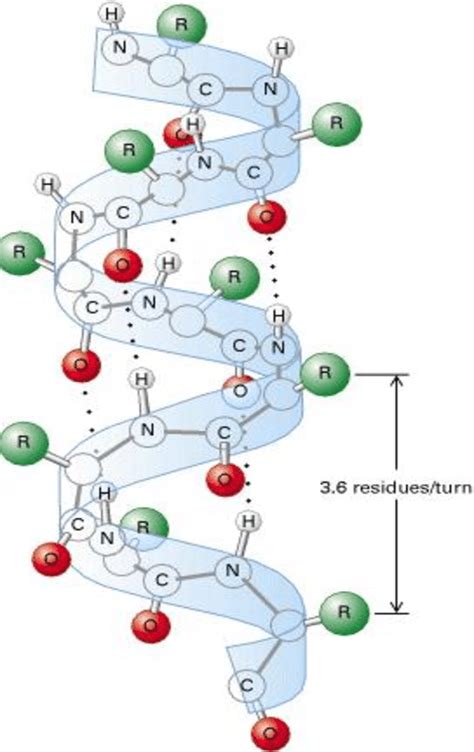 The hydrophobic amino acids fold toward the interiors of proteins (kinda like shown in the α-helix I think)
The hydrophobic amino acids fold toward the interiors of proteins (kinda like shown in the α-helix I think)- The hydrophobic effect is the free energy change of water surrounding a solute
-
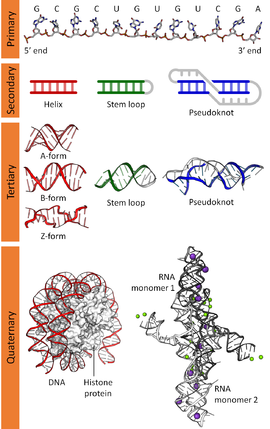
 Nucleic Acids:
Nucleic Acids:
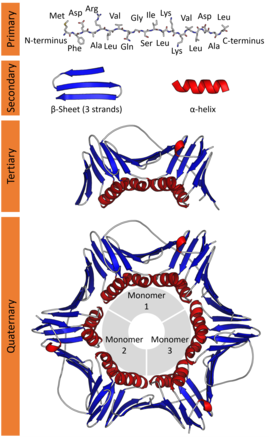
 There’s secondary, tetriary, and quaternary structures for different ’tiers’ of ‘motifs’:
There’s secondary, tetriary, and quaternary structures for different ’tiers’ of ‘motifs’:
Secondary Structures #
- Secondary structures typically spontaneously form before being ‘folded’, due to surrounding Intermolecular Forces.
- They’re defined by the “pattern” of peptide bonds in the backbone, which I think may be independent of the R groups. (maybe the R groups into account makes tert/quater?)
- Peptide backbone refers to the (repeating) structure of the amine and carboxyl as shown in red:
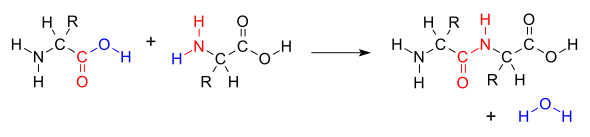 )
)
- Parallel β-sheets:
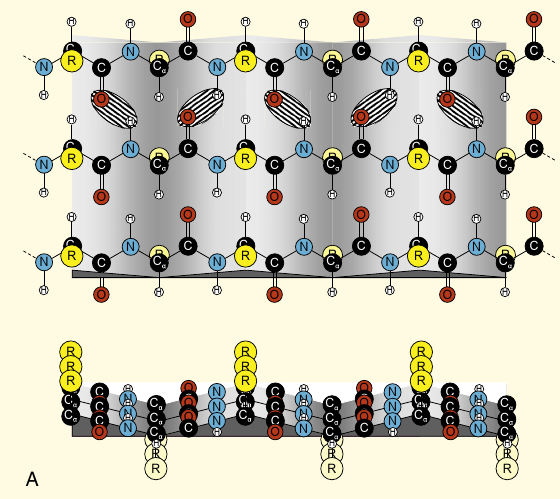 notice how the R groups project laterally. That’s why the chain of NH-CO chains can be called a backbone.
notice how the R groups project laterally. That’s why the chain of NH-CO chains can be called a backbone.
- These must be extremely common with typical α-amino acids, because I don’t exactly see this not always happening if the R groups were not to matter.
- Parallel β-sheets:
Tetriary Structures #
- Tetriary structures are the complete 3d structure of whatever polypeptide.
Quaternary Structures #
- When multiple polypeptide chains assemble they form a quarternary structure.