Intermolecular Force
2022-03-07: reference:
Intermolecular Force #
-
Recall the pictures of the states of matter, and realize that states of matter are no more than intermolecular density. The main physical properties are its volume and shape, that is, if particles are still in contact and if they’re moving.
- It then follows that the stronger the intermolecular force, the higher the boiling point - and the lower the vapor pressure (measured in Pascals - N/m$^2$.)
- Massive or large (a la surface area) atoms/molecules are actually easily perturbable, polarizable, since outer electrons are less attracted to the nucleus because of the shells in between. But notice, that means they’re quite susceptible to London dispersion, which raises their intermolecular force, and thus boiling point, etc.
- It then follows that the stronger the intermolecular force, the higher the boiling point - and the lower the vapor pressure (measured in Pascals - N/m$^2$.)
-
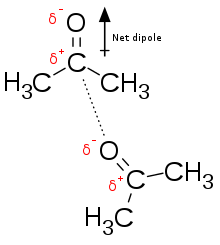 Seeing as many covalent bonds are at least slightly polar, their dipoles have attraction and repulsion to eachother in whatever ensemble they find themselves in.
Seeing as many covalent bonds are at least slightly polar, their dipoles have attraction and repulsion to eachother in whatever ensemble they find themselves in. -
Ion-ion bonding is pretty self-explanatory just the same way. Sometimes by FAR the strongest force as written below
-
Hydrogen bonding… is actually not between hydrogens, but increase when H is bound to N, O, or F. Hydrogen is clearly special in that it only has 1 shell to fill, thus very positively polar, and N/O/F are the most electronegative elements, since they’re compact in shells and when bound, totally full.
- Water is of course interesting in that it’s denser than ice. I guess I assumed being liquid is by definition to be less dense. When solid, water forms a tetrahedral structure with hydrogen bonds:
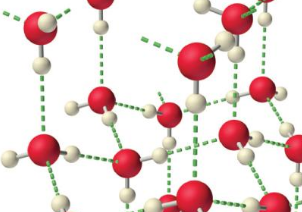 . The liquid is denser because these hydrogen bonds are bound to break.
. The liquid is denser because these hydrogen bonds are bound to break.
- Thus there must be an inherent, independent (relative) variable of some kind of stability as opposed to just density. Should look into this deeper.
- Water is of course interesting in that it’s denser than ice. I guess I assumed being liquid is by definition to be less dense. When solid, water forms a tetrahedral structure with hydrogen bonds:
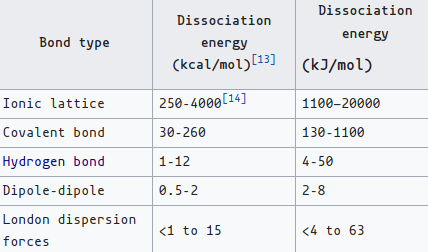
- The van der Waals forces are:
- (Permanent-Permanent) Keesom force: electrostatics; interactions between multiple (rotating) dipoles.
- $\frac{-d_1^2d_2^2}{24π^2ε_0^2ε_r^2k_BTr^6}$, where d = dipole moment, $ε_r$ = dielectric constant, T = temperature, and $k_B$ = Boltzmann constant.
- Hydrogen bonding technically fits under this description though it doesn’t technically fall under the list of ‘van der Waals forces’.
- (Permanent-induced) Debye force: polarization; when one atom’s dipole repels or attracts another previously stable atom’s electrons.
- (Induced-induced) London dispersion force (AKA instantaneous/fluctuation dipole-induced dipole) (or sometimes called THE van der Waals force) is inherent in anything with mass.
- Dipole moment caused by random/inevitable fluctuations of density in a single atom’s (or, of course, a molecule’s) electron cloud, hence instantaneous, especially considering it probably lasts for nanoseconds.
- (Bear in mind atom basically = molecule in these descriptions)
- (Permanent-Permanent) Keesom force: electrostatics; interactions between multiple (rotating) dipoles.
Electronegativity #
-
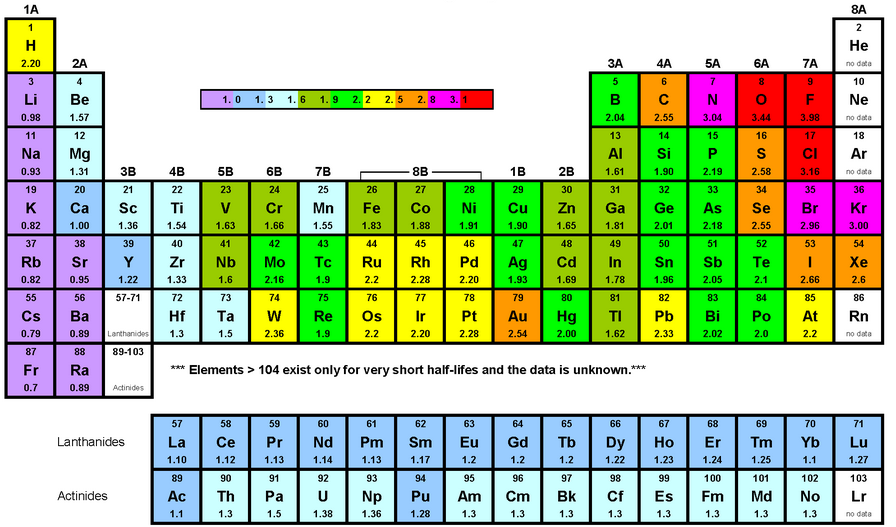
- Electronegativity is itself a property of every element, and oxygen is the 2nd highest in the list. You don’t do much math without first glancing at a chart. Electronegativity is pretty correlated with low atomic number and low valence.
- Hydrogen: 2.0; oxygen is 3.5. Oxygen simply attracts more electrons due to the quantity in its valence shell (combined with its low atomic number), which in H2O gives it a negative charge.
- Sodium is among the lowest electronegativity: as such, it would form extremely weak covalent bonds Happens due to electromagnetism but is not a field of research or anything. Really it’s just a slight detour into more gross chemistry (no not ‘physical’ yet, lol.)
- Electronegativity is itself a property of every element, and oxygen is the 2nd highest in the list. You don’t do much math without first glancing at a chart. Electronegativity is pretty correlated with low atomic number and low valence.
-
Water is the obvious example: the O is much more dense in electrons, thus δ-, negative partial charge. Electrons spend less time revolving around it, and the O attracts more electrons in the bond. A mega simple example is $\ce{\overset{δ+}{H}-\overset{δ-}{F}}$. Within all covalent bonds of different elements there is an assymetric distribution of electrons across the atoms. A difference in electronegativity > 0.4 and it’s considered polar. If the compound is nonionic it obviously balances out overall. Does this have any advanced implications with formal charges? Not sure.