Organic Chemistry
2022-03-24: reference:
- https://chem.libretexts.org/Bookshelves/Organic_Chemistry/Book%3A_Organic_Chemistry_-_A_Carbonyl_Early_Approach_(McMichael)
- https://chem.libretexts.org/Bookshelves/General_Chemistry/Book%3A_Structure_and_Reactivity_in_Organic_Biological_and_Inorganic_Chemistry_(Schaller)
- http://www.probechem.com/ - ultimate ligand search. I saw it in that drug design video
- Drug synth forums (lots of great stickies): https://the-hive.archive.erowid.org/forum/forums.pl, https://chemforum.info/index.php, http://www.sciencemadness.org/talk/
- Determine solubility: http://www.vcclab.org/web/alogps/
Organic Chemistry #
Organic Chemistry with a Biological Emphasis #
- pKa (acid dossication constant) + pKb (base) = 14
1: Structure and Bonding #
Bonding Rules #
- Formal charge = # v.e. of isolated atom - # of nonbonding electrons - 1/2 # of bonding electrons on bound atom.
- i.e. cationic methanol, $\ce{CH3OH2+}$.
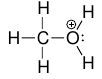 for Oxygen, it’s 6 - 2 - 3 = +1.
for Oxygen, it’s 6 - 2 - 3 = +1.
- i.e. cationic methanol, $\ce{CH3OH2+}$.
- A zwitterion (dipolar ion) is a molecule with equal positive and negative formal charges. Psilocybin for example:
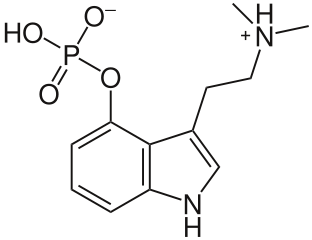
- All Amino Acids when in moderate pH are zwitterions: containing amino ($\ce{-NH^{+}{3}}$) and carboxylate ($\ce{-CO^{-}{2}}$).
- In neutral/alkaline solution (low H+ saturation) they thus become cationic.
- Now’s time to think back to things like -ic acids and how the anionic form is -ate. Take Malic Acid:
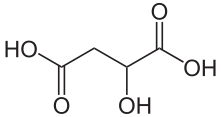 with its two carboxylic acid groups. How do you make this into malate, the anion -2? It’s easy. Same thing for glutamic acid. Same structure.
with its two carboxylic acid groups. How do you make this into malate, the anion -2? It’s easy. Same thing for glutamic acid. Same structure.
- Now’s time to think back to things like -ic acids and how the anionic form is -ate. Take Malic Acid:
Bonding Patterns #
- I think lone pairs imply reactivity.
- Even carbon which is usually tetravalent can have formal charges, and are very high-energy (unstable) when that happens, and are usually very transient intermediates. Carbanions have a lone pair and carbon radicals has a lone “pair” of 1 with the other 3 sides bonded, and is neutral.
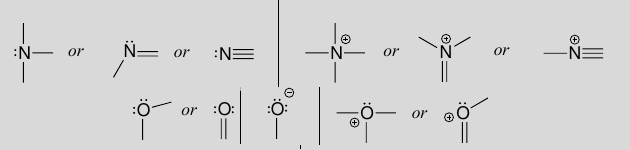
- Sulfur uusually follows the same bonding patterns as oxygen (same group: 6A), despite being in the 3rd row, which the octet rule does not apply to. It can form four or six bonds.
- Phosphorus usually has 3 single bonds and 1 double bond.
- Halogens usually have one bond and three lone pairs, or as monoatomic anions.
Functional Groups #
- Alkanes are saturated hydrocarbons, because they’re bound to the maximum number of hydrogens - while alkenes/alkynes are unsaturated. Hydrogenation is…… the addition of hydrogen atoms, reducing the bonds.
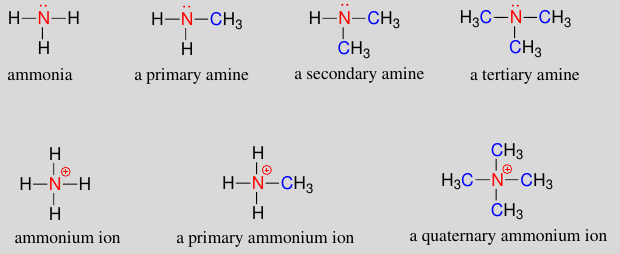 See the pattern ’ere?
Not related but ‘ay, heterocycles:
See the pattern ’ere?
Not related but ‘ay, heterocycles:
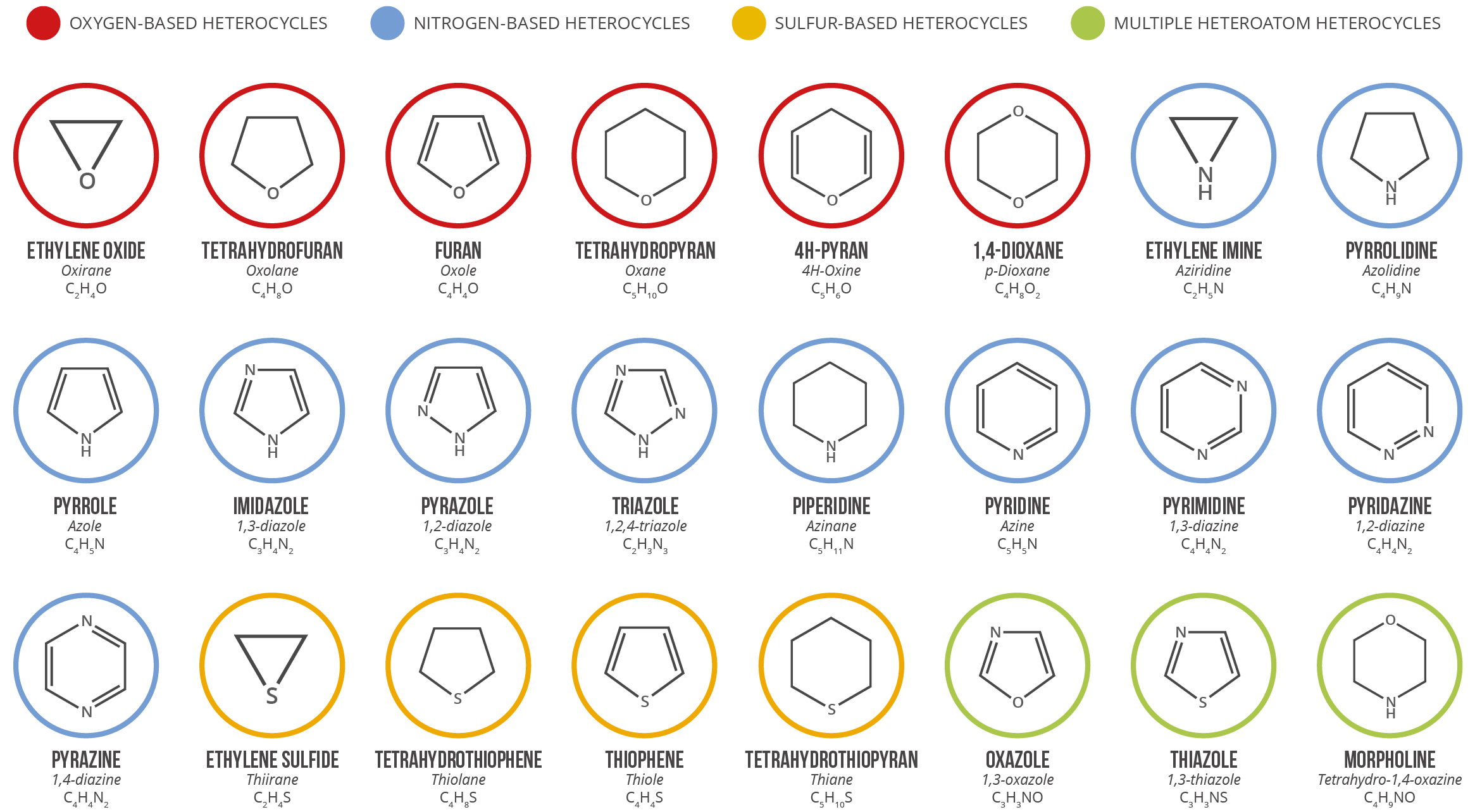
- One thing to keep in mind about these is that the double bonds are not important, but the actual position of the characteristic molecules. In the wild, they can look different.
Nomenclature #
-
The declension is basically wherever the change from the original structure is, and add suffixes accoringly:
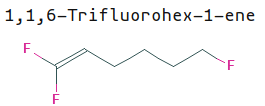
- (While this example breaks this rule, don’t be surprised if you have to count left-to-right)
-
Alcohol: -ol
-
Ketone: -one
-
Ester: -oate, whichever group is attached (to the right)
-
Change -ide to -ic and add hydro- and then acid.
-
Change -ate to -ic and -ite to -ous and then acid.
-
Butanoate = butyrate.
Important Biomolecules #
 ;
;
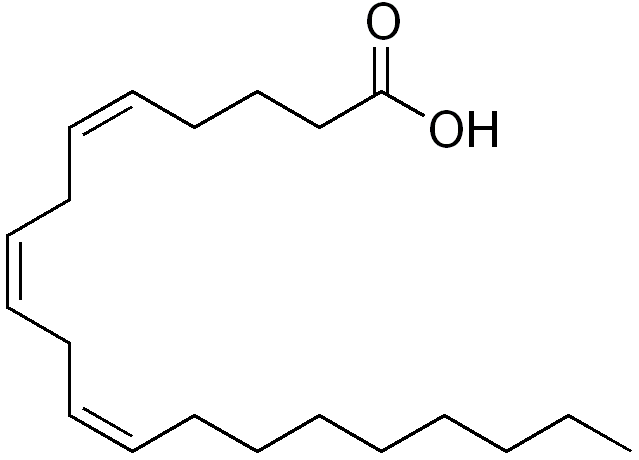
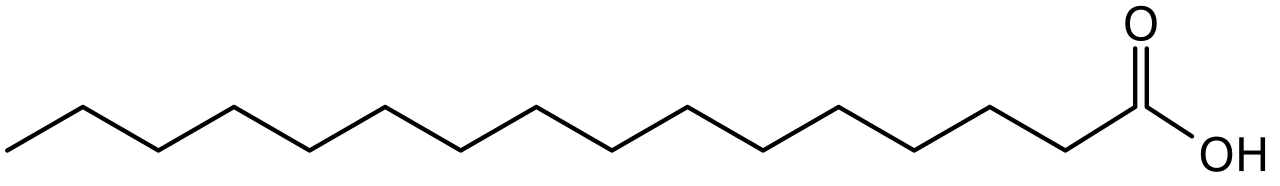 Fatty acids are simply hydrocarbons that terminate in a Carboxyl group. Reminder that saturation is the saturation of hydrogen, that is, the lack of double bonds.
Fatty acids are simply hydrocarbons that terminate in a Carboxyl group. Reminder that saturation is the saturation of hydrogen, that is, the lack of double bonds.
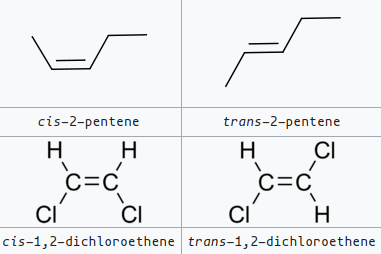
- The double bonds are usually in the cis configuration (L. “this side of”; trans-: “the other side of”):
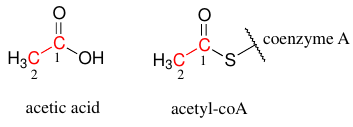
- Fatty Acids are elongated in the body two carbons at a time from the acetate in Acetyl-CoA .
-
-
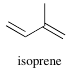 The isoprenoids (terpenoids, as there are mono, di, tri terpenes: iso being n=1) are a class of chemicals, but it seems more like a motif considering how simple it looks and the fact they’re ~60% of known natural products, including steroids and Carotenoids. In eukaryotes, isopentyl diphosphate is the main building block, and is derived from three Acetyl-CoA.
The isoprenoids (terpenoids, as there are mono, di, tri terpenes: iso being n=1) are a class of chemicals, but it seems more like a motif considering how simple it looks and the fact they’re ~60% of known natural products, including steroids and Carotenoids. In eukaryotes, isopentyl diphosphate is the main building block, and is derived from three Acetyl-CoA.
- (I see a pattern here along with Acetyl-CoA: what’s with huge, phosphate-containing molecules being building blocks?)
- ATP is part of A-CoA though. Why not just say that?
- (I see a pattern here along with Acetyl-CoA: what’s with huge, phosphate-containing molecules being building blocks?)
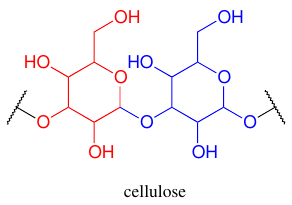
Carbohydrates #
- Saccharides link to other molecules (including to form di/poly) via O-,S-,N-, or C- glycosidic bonds.
2: Structure and Bonding II #
- Catch up on Orbitals, electron configurations, orbital lobes/nodes