@Docosahexaenoic acid membrane properties of a unique fatty acid (Stillwell & Wassall 2003)
2022-03-29: reference:
@Docosahexaenoic acid: membrane properties of a unique fatty acid (Stillwell & Wassall, 2003) #
- The already high DHA levels in these membranes (synaptosomes, sperm, retinol rod outer segment) are not further augmented by diet and once incorporated, DHA is tenaciously retained at the expense of other fatty acids (Salem et al., 1986). - but in other tissues (Phospholipids, mitochondria) it can be enriched 2-10-fold.
- very little DHA-induced al- terations in the fluidity of mitochondrial membranes were reported by Stillwell et al. (1997).
- (Ankified): New incoming DHA prefers Phosphatidylethanolamine over Phosphatidylcholine by about 5.7 times in (phospholipids).
- Salem et al. (1986) have reported that in synaptosomal membranes DHA is found often associated with Phosphatidylserine.
Phase Behavior #
-
In membranes containing DHA the packing is distorted by steric restrictions associated with the presence of multiple rigid double bonds.
- “Rigid double bonds” meaning what exactly? That they’re more rigid than unsaturated at large or that the steric properties within DHA itself makes it so?
 Such that it doesn’t denature into something else?
Such that it doesn’t denature into something else?
- Apparently double bonds are more stronger. BUT, Double bonds are electron-rich, making them reactive in the presence of a strong electron acceptor.
- The reduction in intra- and inter-molecular van der Waal’s interactions produces a decrease in stability. Manifests as a substantial depression in melting transition temperature (Tm) of the gel to liquid crystalline phase transition.
-
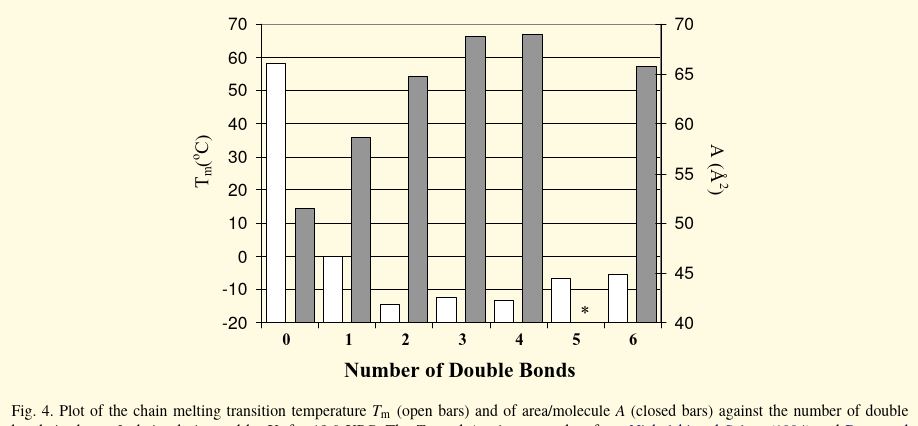
- This is a series of PC with stearic acid (18:0) in the sn-1 and whatever mufa/pufa in sn-2. Not sure what molecules exactly they used besides DHA, (22:6) ALA (18:3ω-3) and AA (20:4ω-6) but I think it may be a grabbag with the sole criteria just being x:n ω-n. The low surface area for 5 tells me it’s probably osbond acid.
-
- “Rigid double bonds” meaning what exactly? That they’re more rigid than unsaturated at large or that the steric properties within DHA itself makes it so?
-
The implied greater stability of the gel state bilayer in the presence of polyunsaturation is particularly marked for 18:0-22:6PC where ΔH Enthalpy = 6.1 kcal/mol is maximal.
Membranes/Fluidity #
-
1,6 Diphenyl 1,3,5 Hexatriene (DPH) is higly fluorescent in lipids - but not water. Thus it is used as a probe in bilayers for measuring phase transitions & fluidity. Fluorescence spectroscopy.
-
The addition of acyl chain double bonds is generally assumed to increase fluidity.
-
*Many dietary studies have reported increases in membrane fluidity from animals fed DHA-rich fish oil diets (e.g. Kamada et al., 1986; Ernst, 1994), while a DHA-deficient diet resulted in a brush border membrane with decreased fluidity (Daveloose et al., 1993). *
- However, as with most dietary studies, the issues are complex and the results contradictory ([Effects of n-3 fatty acids on blood rheology (Ernst, 1999)])
-
Guerre-Millo et al. (1994) isolated the plasma membrane and two internal membranes from metabolically active, genetically obese Zucker rats. While all membranes exhibited an increase in DHA levels, only the plasma membrane showed an increase in fluidity.
-
Numerous studies, nevertheless, did not reveal any significant change in fluidity upon incorporation of DHA, despite using the same techniques (primarily steady state polarization of membrane fluorescent probes) that indicated DHA-induced increase in fluidity in other systems. Dietary studies employing various fish oils as the source of DHA failed to detect changes in the fluidity of Hepatocyte plasma membranes (Clamp et al., 1997), intestinal microvillus (Wahnon et al., 1992), Erythrocytes (Popp-Snijderset al., 1986), platelets (Gibney and Bolton-Smith,10W. Stillwell, S.R. Wassall / Chemistry and1988), and brain synaptosomes (Zerouga et al., 1991).
Permeability #
- Huster et al. (1998) used $\ce{^17O}$ NMR to follow water permeability across lipid bilayers. 18:0-22:6PC membranes are about 4x more permeable than those made of 18:0-18:1 (Oleic Acid)PC but are only about 30% less permeable than those of 22:6-22:6PC (1,2-didocosahexaenoylphosphatidylcholine).
- The wedge shape of DHA-containing Phosphatidylcholines, proposed by Holte et al. (1995) from 2 H NMR, pre- dicts that looser lipid packing at the aqueous interface would result in deeper penetration of water and other solutes into the bilayers. As a result DHA favours increased hydration of the head group and interchain region. The looser surface packing would also favour insertion of proteins into DHA-rich portions of the membrane (Mitchell et al., 1998).
- Brand et al. (1994) made vesicles of phospholipids extracted from liver mitochondria of rats and bearded dragons. They measured H+ leakage and found that vesicles made of lipids from the mammal had a considerably higher DHA content and H+ leakage rate than did vesicles made of lipids from the reptile. Stillwell et al. (1997) corroborated these conclusions by incorporating 18:0-22:6PC into vesicles made from rat liver mitochondrial extracts. ==A linear relationship was noted between H+ permeability and bilayer DHA content.==
- Demel et al. (1972) showed that ==DHA incorporated into the sn-2 position of PC increased permeability to Glucose,== erythritol and Glycerol.
- The increase, however, was lateral pressure-dependent as similar glucose permeability for Oleic Acid and DHA-containing bilayers was observed at high, biologically relevant, pressures.
- When cultured L1210 cells were modified by incubation with DHA or oleic acid, the level of these fatty acids increased to 37% for DHA and 58% for oleic acid. The rate of mitoxantrone uptake in the first minute was 68% greater in DHA- than in oleic acid-supplemented membranes (Burns et al., 1988).