Mucormycosis
2022-06-07: reference:
Mucormycosis #
- It inoculates disrupted skin/mucosa. The causticity to the nose by snorting DFO along with the ferrioxamine is a real double whammy. Thereby it becomes angioinvasive and causes thrombosis.
- Its link to diabetes, apart from dialysis and wounds from insulin injections, is hypergylcemia-induced immunosuppression. I’m not sure what effect insulin has.
- Deferoxamine is itself derived from some kind of bacteria. Ferrioxamine is a siderophore of Rhizopus (iron chelators secreted by microorganisms that help itself accumulate iron to stimulate growth).
- It is asssumed that in clinical setting, the likely portal of entry of disseminated mucormycosis is the respiratory tract. However, experimental mucormycosis by inhalation or by intranasal installation is not possible, except in the diabetic animal.
-
Microbial Siderophore Enterobactin Promotes Mitochondrial Iron Uptake and Development of the Host via Interaction with ATP Synthase
- Enterobactin binds to the ATP Synthase α subunit (it has like a specific binding site), promoting iron uptake.
- It makes sense thatr mitochondria have a specialized iron acquisition system based on siderophore scavening; they used to be free-living bacteria.
- Enterobactin binds to the ATP Synthase α subunit (it has like a specific binding site), promoting iron uptake.
-
Isolated Fungal Sphenoid Sinusitis With Cavernous Sinus Thrombophlebitis: A Case Report
- Treated with itraconazol whereby everything reverted? Rhino-orbital-cerebral muomyosis associated with (ketoacidotic) diabetes, immunocompromised states, renal disease, deferoxamine use and acidotic states.
- Patient taking DFO for hemochromatosis have been a predisposing factor in developing mucormycosis (black fungus in the nose, sinus eye, and eventually brain)
R.
- Mucor is the opportunistic angioinvasive fungus. It innoculates nasal mucosa
- vascular occlusion, consequently resulting in extensive tissue necrosis
- Mucor is the opportunistic angioinvasive fungus. It innoculates nasal mucosa
-
Mucormycosis during Deferoxamine Therapy Is a Siderophore-mediated Infection
- Fe.DFO, the iron chelate of DFO,(ferrioxamine?), abolishes the fungistatic effect of serum on Rhizopus and increases the in vitro growth of the fungus. Present at Fe.DFO concentrations >=0.01 μM, at which fungal uptake of radioiron from $\ce{^55Fe}$.DFO (from Fe=26) is observed.
- DFO has a molecular mass of 560g/M. 250mg dose = 2.24 μM?
- Fe.DFO, the iron chelate of DFO,(ferrioxamine?), abolishes the fungistatic effect of serum on Rhizopus and increases the in vitro growth of the fungus. Present at Fe.DFO concentrations >=0.01 μM, at which fungal uptake of radioiron from $\ce{^55Fe}$.DFO (from Fe=26) is observed.
-
Presentation and outcome of rhino-orbital-cerebral mucormycosis in patients with diabetes FREE
- external ophthalmoplegia (89%), proptosis (83%), visual loss (80%), chemosis (74%), and eye lid gangrene (14%). Non-ophthalmic manifestations included sinusitis (100%), nasal discharge/ulceration (74%), infranuclear VI nerve palsy (46%), palatal necrosis (29%), cerebral lobe involvement (20%), and hemiparesis (17%). Computed tomography/magnetic resonance imaging showed involvement of paranasal sinuses in all patients with ethmoid (86%) and maxillary (80%) sinuses being most frequently involved.
-
Rhinocerebral mucormycosis during deferoxamine therapy
- Myeloid leukaemia requierd transfusions with 5g of deferoxamine after each transfusion. Took 7 months of however frequent this was.
- Phlegmon developed within a few days with protrusion and blindness of the left eye necessitating a decompression operation. Material obtained at operation revealed rhinocerebral mucormycosis. After 3 weeks of antimycotic treatment with both amphotericin B (1 mg/kg.d) and flucytosine (150 mg/kg.d) the mucormycosis healed without the necessity of extensive and disfiguring removal of necrotic tissue. But the blindness in the left eye, caused by occlusion of the central artery, was irreversible.
-
Epidemiology and clinical manifestations of mucormycosis.
- Third most important mycosis after candidasis and aspergilosis. Agents include the zygomycetes: Rhizopus, Rhizomucor, Apophysosomes, Mucor, Cunninghamella, Lichthemia, Saksenaea. Some do not produce deferoxamine but do utilize it, like Streptomyces pilosus, Yersina enterocolitica and Vibrio vulnificus.
- Occasionally, cerebral vascular invasion can lead to hematogenous dissemination of the infection.
-
Mucormycosis: Association with Deferoxamine Therapy
- Associated with acute leukemia. Leukemia is a cancer of the blood/bone marrow
- Transfusion-related iron overload in patients undergoing dialysis predisposes these patients to bacteremia with Yersinia and Listeri
-
Iron uptake from ferrioxamine and from ferrirhizoferrin by germinating spores of Rhizopus microsporus
- It is concluded that iron accumulation by Rhizopus from Fe.DFO is an energy-requiring process, which involves dissociation of iron from the Fe.DFO complex and which could require a reductive step. Apo-DFO returns to the extracellular medium(/plasma?)
- No iron transfer takes place between Fe.DFO and apo-transferrin
- Logarithmic curve on iron uptake without human serum:
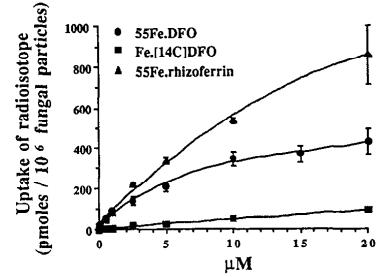 .
.
- Like apparently the Rhizopus accumulates 14C? It is linearly proportional to chelator concentration.
- BUT THEN, when 40% human seurm is added and it is place in BDM, which replicates mammalian cells I guess, it becomes basically perfectly proportional to uptake.
- Fe.DFO and Fe.Rhizoferrin competitively inhibit the uptake of the other, but this isn’t relevant as ingesting the rhizoferrin is suicide.
- more inhibited than fungal growth. Bipyridyl, a chelator of Fez’+, also inhibits both the iron accumulation and the growth of the fungus induced by Fe.DFO (Fig. 9). The effect of bipyridyl is concentration dependent.
- In 30 minutes, Fe uptake basically fully accumulates.
-
Mucormycotic slough of nasal floor and palate in the anephric patient
- Nasal involvement is indicated by a dark, blood-tinge discharge, often mistaken for dried blood. Early necrosis of the septum and turbinates is common.
- The frontal and·ethmoid sinuses are rarely involved, but the maxillary sinus shows nodular thickening and a lack of fluid level
- Acidosis is often the common denominator, in which state Mast Cell function is inhibited and thus a poor response to inflammation.
- The concomitant use of antibiotics, steroids, and radiation are thought to accelerate fungal growth.
Antifungals #
All in all, posaconazole works, but I don’t think any of those side effects make it worth using as a preventative measure, especially over something like rapamycin. If high dose rapa doesn’t work and I get some kind of obvious symptoms, then I’m off to the ER brotha!
-Conazol #
- Posaconazole is an option typically used as “step-down therapy”. Or IV as salvage therapy for those who cannot tolerate or respond well to AB, which is 300mg every 12 hours on day 1, and 300mg every day after. Cyclodextrin vehicle accumulation is possible in the kidneys so IV is avoided in those with renal impairment.
- Requires fatty food for oral absorption. Possibly relevant for intranasal use (I don’t think that’s a good idea though)
- Posaconazol has questionble effectiveness. However, it is quite safe and nontoxic, for months. R
- Seems legit bro: https://www.indiamart.com/proddetail/picasa-40-mg-suspension-20092461055.html?pos=13&kwd=posaconazole&tags=B… it’s $151. There’s another bottle for sale for like 1/10 the price for some reason: https://www.indiamart.com/proddetail/posaconazole-syrup-or-oral-suspension-23622328355.html?pos=11&kwd=posaconazole&tags=B. If this isn’t some blatant scam I’ll go with this one.
- Naturally, at mayoclinic.org, it has every potential side effect under the sun. including tarry stool, seiures, and fruity breath. The hell man? I wonder if this is at all relevant were a healthy person to take it. Honestly that’s probably a lot of drugs though.
- reported in vitro minimum inhibitory concentration for 90% of organisms (MIC90) of posaconazole against the Mucormycotina has ranged from 1 μg/mL to ≥4 μg/mL. However, in patients with febrile neutropenia or invasive fungal infections, posaconazole dosed at 400 mg twice daily resulted in serum levels less than 1 μg/mL
- Requires fatty food for oral absorption. Possibly relevant for intranasal use (I don’t think that’s a good idea though)
- Isavuconazole: 200mg oral/IV every 8 hours for the first 6 doses, then 200mg every 24 hours.
Amphotericin #
-
Cerebral mucormycosis: intranasal route to deliver amphotericin B for effective management?
- ~5mg/kg IV. For like 60-100 days??
- Other anti-fungal compounds such as voriconazole, fluconazole, and itraconazole do not depict reliable activity against mucormycosis, even though voriconazole has the ability to cross the blood-brain barrier. In a previous study, it was shown that voriconazole prophylaxis is a risk factor for mucormycosis
- Amphotericin B seems to be the main thing in studies. It binds to ergosterol, creating a channel, and destabilizes the membrane/biofilm, leading to cell rupture, cell death, oxidative damage, etc. Effective against candida (especially albicans) and many other things.
- An elimination/terminal half-life of approximately 15 days follows an initial plasma half-life of about 24 hours.
- Nephrotoxic (kidneys) due to vasoconstriction and them, and a lytic action on cholesterol-rich lysosomal membranes of renal tubular cells.
- Pubchem page give an absolute pile of side effects.
Rapamycin #
-
Rapamycin Exerts Antifungal Activity In Vitro and In Vivo against Mucor circinelloides via FKBP12-Dependent Inhibition of Tor
- While posaconazole is not synergistic with amphotericin, rapamycin is - wither either, that is.
- Tarcolimus, AKA FK506, nuked it at the dose they used compared to rapa.
-
Antifungal drug resistance evoked via RNAi-dependent epimutations
-
Broad antifungal resistance mediated by RNAi-dependent epimutation in the basal human fungal pathogen Mucor circinelloides
- Our previous work demonstrated that one major agent of mucormycosis, Mucor circinelloides, can develop resistance to the antifungal agents FK506 and rapamycin through a novel, transient RNA interference-dependent mechanism known as epimutation.
-
Broad antifungal resistance mediated by RNAi-dependent epimutation in the basal human fungal pathogen Mucor circinelloides
Treatments #
- 50mg/kg in mice. HED 250mg-500mg.
- Pax does 250mg per mL of insulin and takes 100 IU/day.
- Sigmaaldritch says 50mg/mL in water.
- Intranasal deferoxamine chelates 15% of the systemic iron of IV deferoxamine and has much lower systemic exposure. The levels that reach the frontal cortex are 271x higher. Hanson et al 2009 showed 10x higher.