Drugs
links: reference:
- https://en.wikipedia.org/wiki/Lysergamides#References Has a massive list of probably all drugs imaginable at the bottom.
- https://drughunter.com/
- Drug interactions: https://supp.ai/
- https://labs.icahn.mssm.edu/maayanlab/ has a tool: https://maayanlab.cloud/sigcom-lincs/#/SignatureSearch/UpDown where you set genes to upregulate/downregulate and it matches drugs that effect them.
- https://www.buy-pharma.md/ legit according to Uxkull etc.
- Morphinan History X - Molecusexuality of Opioid Stereochemistry: The Morphinan In the Mirror, Part I - A well cited exploration into the Stereochemistry, Geometry and Sterics of the Opiosphere - by Dμchess Vσn δ + the “Notorious Gibbs Free Energy” 4-20-2021
Drugs #
My pharmacopoeia in the links tab… not sure how helpful that actually is.
- DREADDs: “Designer Receptors Exclusively Activated by Designer Drugs”: DREADDs for Neuroscientists
Drug Discovery #
- Drug discovery $\approx$ medicinal chemistry.
- SMILES: simplified molecular-input line-entry system. A way to write molecules in terms of a string of text, e.g. Cn1cnc2c1c(=O)n(c(=O)n2C)C: caffeine. It’s usually on a drug’s wikipedia page
- Allosteric Modulator Leads Hiding in Plain Site: Developing Peptide and Peptidomimetics as GPCR Allosteric Modulators
-
https://www.molecular-modelling.ch/swiss-drug-design.html - Swiss Institute of Bioinformatics: it has this molecular drawing frontend called Marvin JS by ChemAxon and then a few sites for a few tasks:
- Predict targets from structure: http://www.swisstargetprediction.ch/
- Predict ADME parameters (absorption, distribution, metabolism, and excretion), pharmacokinetics, etc. http://www.swissadme.ch/
- Predict interactions between a molecule and a protein: http://www.swissdock.ch/
- Find isosteres:
http://www.swissbioisostere.ch/
- An (bio)isostere is a molecule or group with similar shape and usually electronic properties to another.
- Maybe this is the basis of drug design. I just know that non-alkaloids don’t survive the test of time in the body for some reason. But I think this is more for the sake of creating slightly different effects while maintaining its ADME properties.
- A ’non-classical’ isostere is more on the side of preserving the needs of a ligand in question without much concern for gross structural similarity.
- For instanced, replacing a hydrogen with a fluorine can lengthen the half-life by preventing oxidation.
- They’re thinking about instead of patenting just molecules, they’ll patent the ‘pharmacophore’, the 3D arrangement of forces and features and stuff:
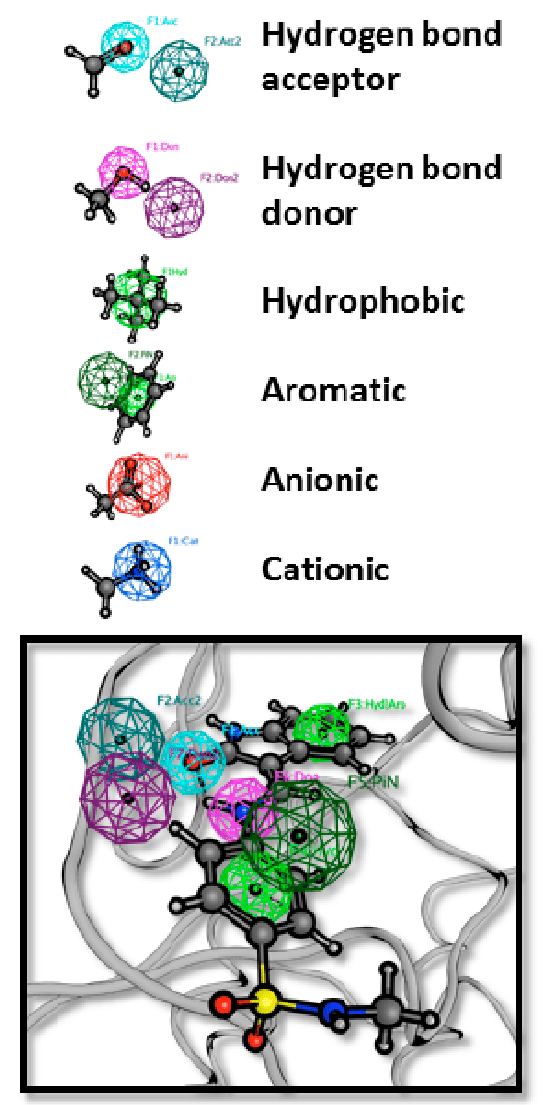
- An (bio)isostere is a molecule or group with similar shape and usually electronic properties to another.
- Find similar molecules: http://www.swisssimilarity.ch/
- Similarity ensemble approach
https://sea.bkslab.org/
- E.g. NSI-189:
https://sea.bkslab.org/jobs/search_cd77034f-7661-47d4-af3d-64b4c8c37eb5
- We’ve got FAAH, α1, AChE, and some other stuff
- E.g. NSI-189:
https://sea.bkslab.org/jobs/search_cd77034f-7661-47d4-af3d-64b4c8c37eb5