Retina
2022-10-01:
Retina #
- Btw, JC is selling the eye lightening drops for free alongside orders of the other drops.
Color Vision #
-
-
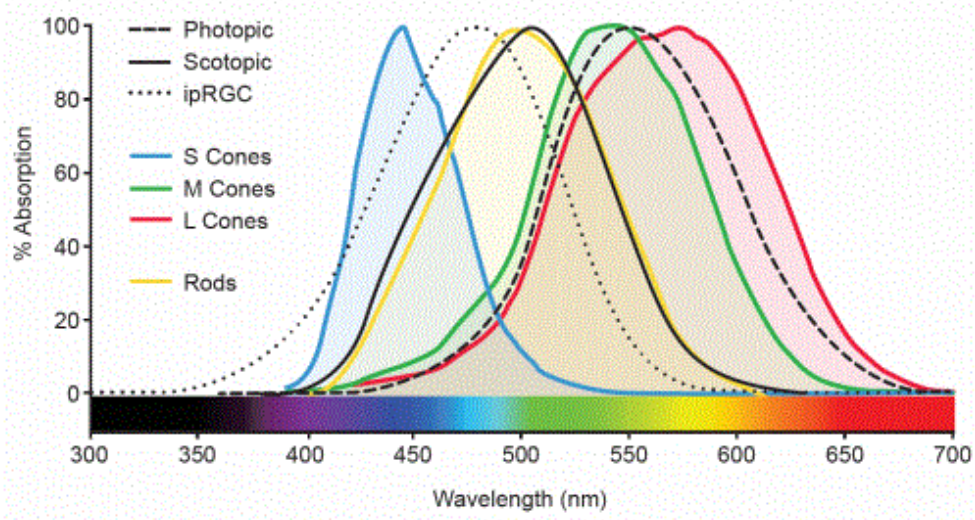
- These are actually just the α-bands of the cones. There’s a β-band that extends into the UV range that the lens of the eye blocks, and they all pretty much overlap, leading to white.
-
YT: Colour Mixing: The Mystery of Magenta
- Indeed, for additive color mixing, yellow and cyan are found between their constituent colors (red-green and blue-green) but notice magenta (red-blue) is in an odd spot: it doesn’t make green. $\text{\color{magenta}Magenta}$ is the ‘absence of green’ or something; it has no spot on the electromagnetic spectrum.
-
-
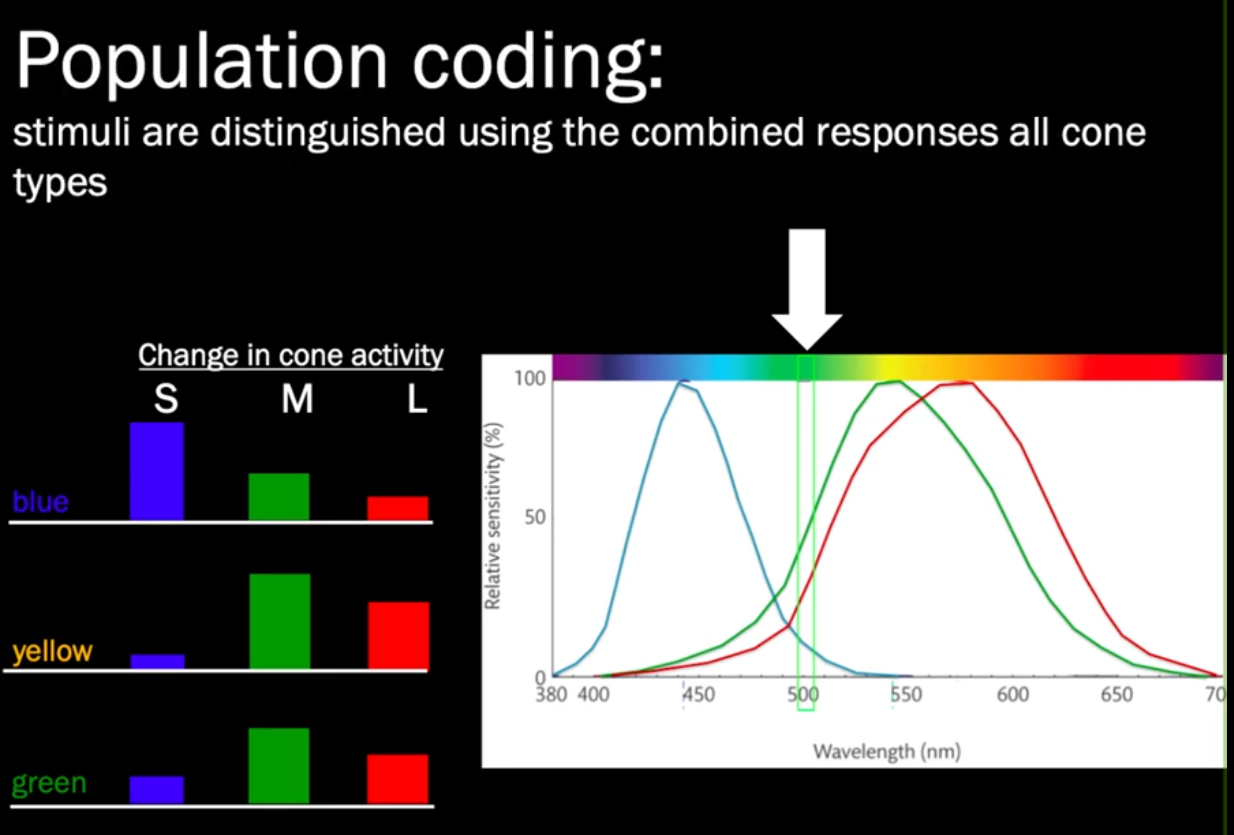
Opsins are proteins (GPCR?) that are made photosensitive with retinal. The three cones in humans are $\color{red}OPN1LW$, $\color{green}OPN1MW$, and $\color{blue}OPN1SW$ (= long, short, medium wave (sensitive)), or 557-575, 527-535, and 420-445 nm respectively.
- Notice 575nm is not in the red. The signals aren’t the literal wavelengths but relative differences computed by post-receptoral cells in the retina and brain.
- Notice how red and green are closer than green to blue; the deficiency in green for deuteranopia, for instance, leads to lack of discrimination between red as well, thus the blue-yellow vision.
-
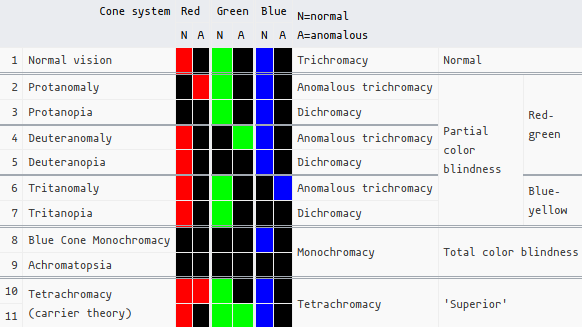
- N = normal, A = ‘anomalous’, which I think is just some kind of deficiency, i.e. lack of receptors or lack of sensitivity, rather than blindness/absence.
-
There exists conditions such as cerebral achromatopsia, which is the ability to see color, but the inability to ‘recognize’ them, due to damage to the cerebral cortex.
-
Intrinsically photosensitive retinal ganglion cells contain melanopsin OPN4. There’s also OPN5 contained in neural retina and the RPE, and it has an λmax of 380 nm.
-
#Ankified Dichromats have a ’neutral point’ where light appears achromatic. Anything below that is blue, anything above that is yellow, corresponding to the 2 remaining cones. The neutral point, being achromatic/gray is equal sensitivity between the 2.
- Protanopes are 495 nm, deuteranopes are 500 nm.
Genetics #
- $\color{red}L$- and $\color{green}M$- type opsin are only found on the X chromosome (and color blindness is recessive). Therefore it’s much more common in males, as this is corrected in females before lyonization. Either the gene is missing, or the protein is rendered dysfunctional. Meanwhile, $\color{blue}S$ is found on chromosome 7.
- The most common form of inherited color blindness is deletion of these photoreceptors.
- The structure of the OPN protein actually directly influences the peak sensitivity of 11-cis retinal, by squeezing it somehow.
- According to OMIM and G-proteins | Color Vision, whereas there is a single red pigment gene, green pigment genes vary in number among persons with normal color vision, with $\color{green}OPN1MW$ being a repeated sequence. This repetition increases the likelihood of gene rearrangements, even though only the first two repeats are expressed and contribute to the color vision phenotype.
-
A thyroid hormone receptor that is required for the development of green cone photoreceptors TRβ2 is expressed on the outer nuclear layer of the embryonic retina. Deletion causes selective loss of $\color{green}M$-cones and concomitant increase in $\color{blue}S$-opsin cones.
- I only imagine knockout causes other major defects.
-
Epigenetic control of expression of the human L- and M- pigment genes
- OPN1LW and OPN1MW are rich in CpG dinucleotides which 200 bases subject to epigenetic regulation (methylation), which promotes chromatin condensation -> gene silencing.
-
The molecular basis of dichromatic color vision in males with multiple red and green visual pigment genes (Jala 2002) - this is rich.
-
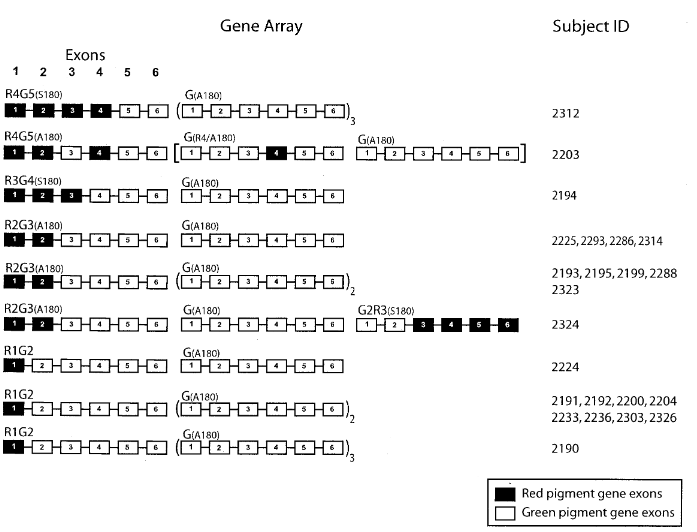
- Of the 27 deuteranopic subjects, 23 had arrays comprised of one normal R-pigment gene (occupying the first position), at least one G/R-hybrid gene, and one or more (up to six) normal G-pigment genes… Five different types of G/R-hybrid gene were identified… Among these 23 arrays, 65% contained a G3R4(A180) hybrid gene paired with a R(A180)-pigment gene in the first position. This pair of genes encodes identical or nearly identical pigments.
- In two of the 27 subjects, deuteranopia was not caused by the presence of a G/R-hybrid gene, but by C203R mutation in exon 4 of their most proximal (upstream) G-pigment gene… C203R was also present in one of the G-pigment genes of a third subject who also possesses a G2R3(S180) hybrid gene in his array.
- Two other subjects had neither a hybrid gene nor C203R, and no other mutation was indentified as the cause!
-
-
The molecular basis of variation in human color vision (Deeb 2005)
- Goes deep into the hybrid genes.
Gene Therapy #
- CRISPR/Cas9—A Promising Therapeutic Tool to Cure Blindness: Current Scenario and Future Prospects (2022)
- The star of the show:
Gene therapy for red-green colour blindness in adult primates (Manusco et al. 2009)
- Red-green color blindness, “Daltonism,” is the most common single locus genetic disorder, affecting 6% of men worldwide
- I worry that perhaps they got enhanced color discrimination in the deuteranope range, allowing them to see “red” by being a different shade? Or is that now how it works.
- I need to check, but I think the 20-week mark when they were suddenly able to discriminate, may have been how long it took for the cones to merely be expressed.
- [Gene therapy in colour (2009)]
- All male squirrel monkeys are dichromats, while most females are trichromats.
- Specifically, male squirrel monkeys have an $\color{blue}S$-cone, and an $\color{green}M$-cone that ends up being either with 535, 545, and 560. 560 (M3) is of course like the human $\color{red}L$-cone.
- Injecting a virus with the gene for the missing photopigment does indeed confer trichromatic vision in adults after 20 weeks. Almost simultaneously with the appearance of viable new photopigment, the formerly dichromatic monkeys became able to perform the colour-discrimination task as proficiently as trichromats. That there was no measurable delay in visual function suggests that the new cone signals were combined immediately in pre-existing colour channels from eye to brain.
- It took 20 weeks for stuff like GFP to show up too apparently: Recombinant adeno-associated virus targets passenger gene expression to cones in primate retina (2007)
- They targeted it preferentially to M-cones, which produced a relatively uniform, third submosaic of approximately 15–36% of M cones that coexpressed the transgene
- Cone-specific expression using a human red opsin promoter in recombinant AAV (different lab btw)
- A priori, there were two possibilities for how a change in spectral sensitivity might change colour vision behaviour:
-
- animals may have an increase in sensitivity to long-wavelength light, but if the neural circuitry for extracting colour information from the nascent “M+L cone” submosaic was absent, they would remain dichromatic, the hallmark of which is having two hues that are indistinguishable from grey
-
- The second, more engaging possibility was that treatment would be sufficient to expand sensory capacity in monkeys, providing them with trichromatic vision. In this case, the animals’ post-therapy results would appear similar to Fig. 2e, obtained from a trichromatic female control monkey.
- As shown in Fig. 3b and c, both monkeys’ measured thresholds for these additional hues were similar to their thresholds for DW = 490 nm, demonstrating they now lacked a spectral neutral point and have become truly trichromatic. Furthermore, treated monkeys were able to discriminate blue-green (DW = 490 nm) when it was tested against a red-violet background (DW = −499 nm), instead of the grey background, indicating that the monkeys’ newly-acquired “green” and “red” percepts were distinct from one another.
-
- For example, L and M opsin-specific genetic regulatory elements might have been required to direct the opsins into distinct cone types ( Molecular genetics of inherited variation in human color vision (1986)) that would be recognized by L and M cone-specific retinal circuitry ( Specificity of Cone Inputs to Macaque Retinal Ganglion Cells (2008)), and to account for cortical processing, multi-stage circuitry ( A multi-stage color model (1993)) might have evolved specifically for the purpose of trichromacy. However, our results demonstrate that trichromatic colour vision behaviour requires nothing more than a third cone type
- All male squirrel monkeys are dichromats, while most females are trichromats.
-
http://www.neitzvision.com/research/gene-therapy/ they use “we” when referring to the research. So they’re affiliated with Manusco and whatnot. One of the researchers on the paper uploaded the Youtube videos
- There is not an equal mosaic:
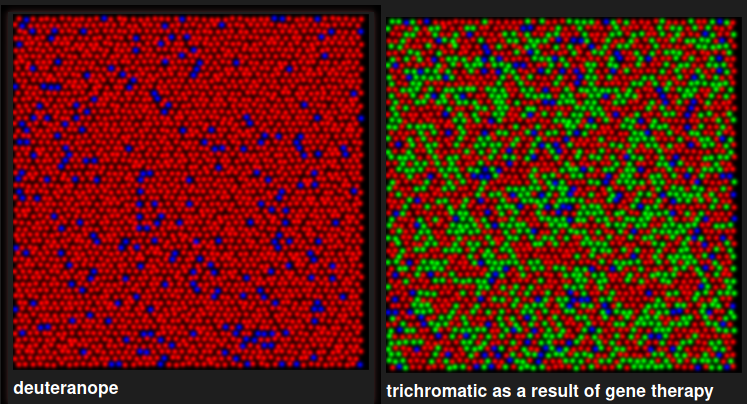 notice that OPN1SW only makes ~5-7% of the cones in mammals, which is pretty interesting—I bet this makes up for the disproportionately lower wavelength (since OPN1MW/LW are only 30nm apart but OPN1SW is almost 100 lower than OPN1SW.)
notice that OPN1SW only makes ~5-7% of the cones in mammals, which is pretty interesting—I bet this makes up for the disproportionately lower wavelength (since OPN1MW/LW are only 30nm apart but OPN1SW is almost 100 lower than OPN1SW.)
- (This could be a cool in silico simulation! Information Technology)
- There is not an equal mosaic:
Mutations #
-
An A-71C substitution in a green gene at the second position in the red/green visual-pigment gene array is associated with deutan color-vision deficiency
- In 32 of 37 (86.5%) Japanese subjects with deutan colorblindness (CBD; 303800) and a normal genotype, Ueyama et al. (2003) identified a -71A-C transversion in the green pigment gene at the second position in the array. The diverse phenotypes in subjects with the -71C promoter change, ranging from the most severe form (deuteranopia, present in 5) to the mildest form (pigment color defect, present in 8), was unexplained
- C203R: Missense mutation; replacement of cysteine with arginine at position 203, due to an SNP: position 1101C->T. Since OPN1MW and OPN1LW are 98% homologous, (S is ~40%).
- [The importance of deleterious mutations of M pigment genes as a cause of color vision defects]
- Deuteranopia is often caused by the loss of M genes, but here we report that it can also be caused by missense mutations that interrupt the function of all M pigments.
- Eighteen were estimated to have a single L pigment gene on the X-chromosome. In most cases, the one L gene was the only X-linked photopigment gene, as has been reported previously for deuteranopes. However, four of the boys in this group were estimated to have M pigment genes in addition to a single L.
- Sequences of exons 2-4 of the M genes were obtained from two of the subjects whose ratios indicated one L and two M genes. C203R was found.
- [The importance of deleterious mutations of M pigment genes as a cause of color vision defects]
- [Structural and functional alterations associated with deutan N94K and R330Q mutations of green cone opsin.]
- N94K
- R330Q: Reduced transducin activation activation ability.
Tetrachromacy #
- Human tetrachromats possibly have ‘OPN1MW2’ or which is just a duplication of OPN1MW. It’s just a theory though, I think.
- Zebrafish and goldfish are natural tetrachromats, having an additional cone for the ultraviolet range.
- https://en.wikipedia.org/wiki/Tetrachromacy?useskin=vector#Humans
Purkinje Effect #
- (Not related to Purkinje cells)
- There’s something of an inverse relationship between color vision and luminance-sensitivity, shifting towards the blue end of the spectrum, since rods are more sensitive to green/red. The two photoreceptors are cone (photopic; bright) and rod (scotopic; dim) repsecitvely.
Anatomy #
- The mascula is this yellow pigmented area in the center of the retina, which absorbs excess UV-blue light.
-
Phagocytosis by the Retinal Pigment Epithelium: Recognition, Resolution, Recycling
-
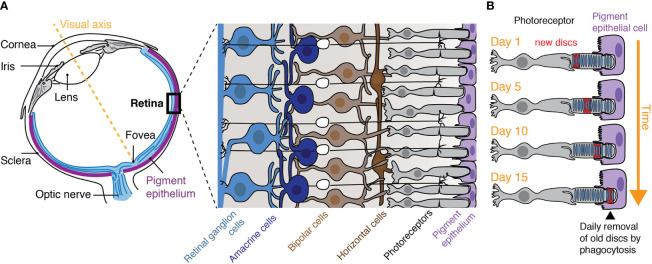
- The RPE that underlies the retina is responsible for the turnover of photoreceptor outer segments (POS) by their phagocytosis and is critical for photoreceptor function
-
-
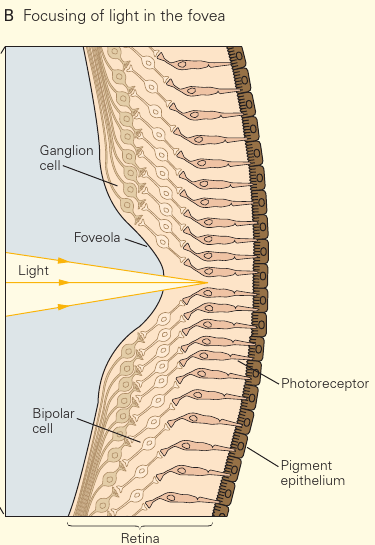 Notice how all the ganglia are shifted to the side in the foveola. This is so that light has direct access to the photoreceptors, in order to reduce blur and light scattering. #Ankified
Notice how all the ganglia are shifted to the side in the foveola. This is so that light has direct access to the photoreceptors, in order to reduce blur and light scattering. #Ankified
- S-cones are absent from the fovea
- At night, the central fovea is blind owing to the absence of rods. Astronomers know that one must look just to the side of a dim star to see it at all. During nighttime walks in the forest, we nonastronomers tend to follow our daytime reflex of looking straight at the source of a suspicious sound. Mysteriously, the object disappears, only to jump back into our peripheral field of view as we avert our gaze.
- P-type ganglion cell (aka midget retinal ganglion cell) = projects to the parvocellular layers of the lateral geniculate nucleus.
- Slow conduction velocity. Receive input from relatively few rods/cones. Responsive to changes in color, but not so much contrast. Center-surround receptive fields.
-
Retinal receptive-field substructure: scaffolding for coding and computation
-
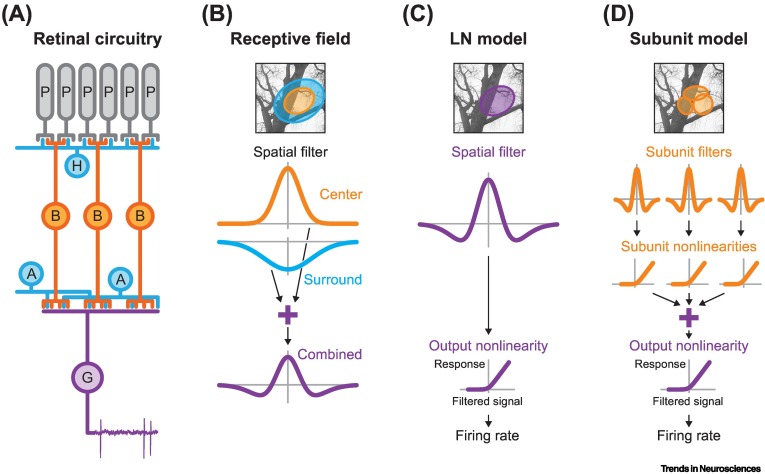
- Depending on the cell, the center field can either be ON or OFF, with the surround being the opposite.
-
-
Retinal receptive-field substructure: scaffolding for coding and computation
- Slow conduction velocity. Receive input from relatively few rods/cones. Responsive to changes in color, but not so much contrast. Center-surround receptive fields.
- M-type projects to the magnocellular layers of the lateral geniculate nucleus**
- Fast conduction velocity. Receive input from a large number of rods/cones. Not very sensitive to changes in color. Larger receptive fields than P-type.
Phototransduction #
- This is important for the ‘wrap your head around’ part: Cone cells compare their signal to the surrounding ones, and sends a light/dark signal accordingly.
Jay Neitz: Color Vision, Molecular Genetics
- I think this is just the opponent process?
- Y-b = stimulated by yellow, inhibited by blue.
- r-g = stimulated by red, inhibited by green.
- w-bk = stimulated by white, inhibited by black.
- I think this is just the opponent process?
- Kandel chapter 22 covers a lot.
-
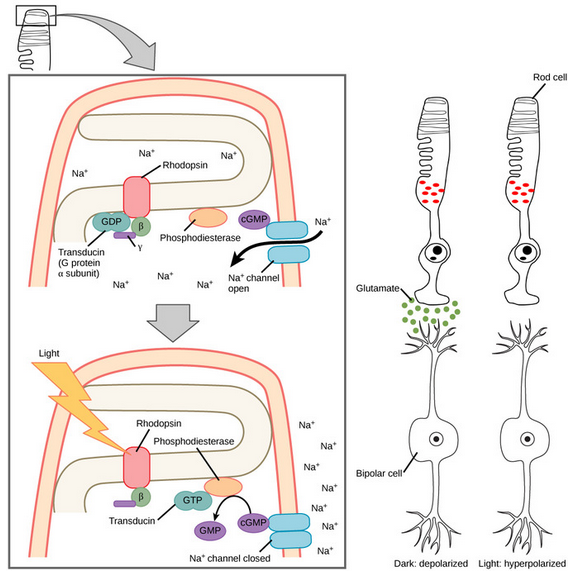
- So isomerization → Opsin activates PDE → cGMP closes sodium channel → Hyperpolarization, action potential.
- Each retinal ganglion cell gets signal from ~24 different cones. They have a receptive field, where closer cells produce a more salient stimulus (i.e. faster firing rate)
- for a given ganglion cell, its receptive field would enclose all the synapsing network of photoreceptors, bipolar, horizontal and amacrine cells that converge to it.
- So isomerization → Opsin activates PDE → cGMP closes sodium channel → Hyperpolarization, action potential.
-
https://en.wikipedia.org/wiki/Principle_of_univariance?useskin=vector:
this video explains it pretty well. But basically, the same cone can be stimulated by a variety of completely stimuli. Bright green and dark blue essentially elicit the same response.
-