Photosynthesis
2022-08-26: reference:
Photosynthesis #
- $\ce{6CO2 + 6H2O ->[Sunlight] C6H12O6 + 6O2}$
- Notice how there’s no investment in energy, unlike animal cells. This is what “producing their own food” means, even though it’s technically thanks to the sun.
- $\ce{CO2 + 2H2A->[Light] CH2O + 2A + H2O}$ where A is the electron donor, aka or oxygen - or water.
- This is the real skeleton of this simple reaction. Some green/purple sulfur bacteria use sulfur or hydrogen. Some nonsulfur bacteria use amino/organic acids.
- If you count the valence electrons you get: 6(16) + 6(8) ⟹ 54 + 6(12) = 144 ⟹ 126. These 18 missing electrons are what comes from the sun (or from 36 photons/36hν).
- It seems the physiology is somewhat analogous to the electron transport chain:
-
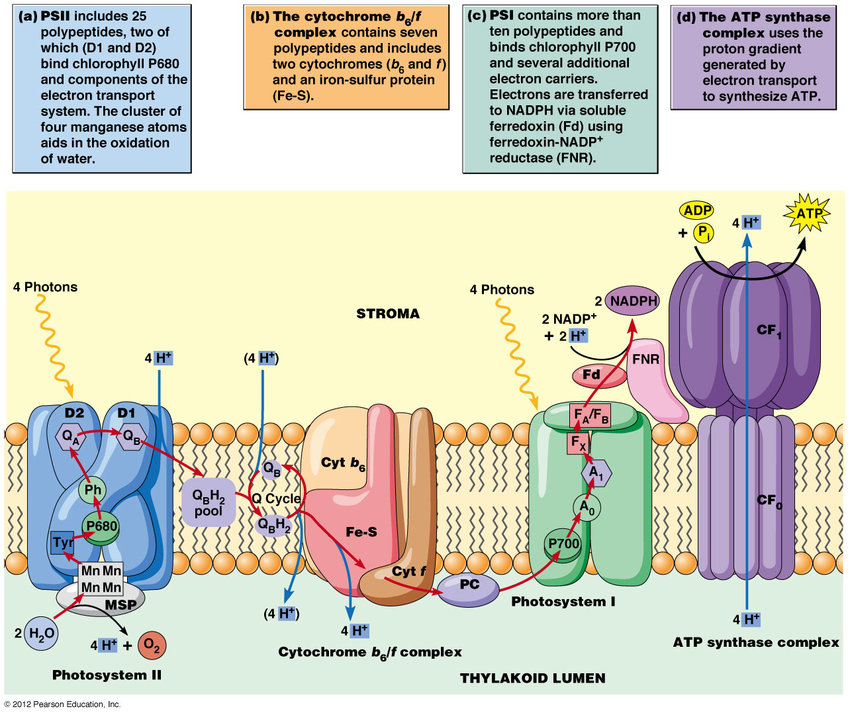
- Source: Absolute madness with like 68 figures. Biotechnological development of the marine microalgae Nannochloropsis gaditana
-
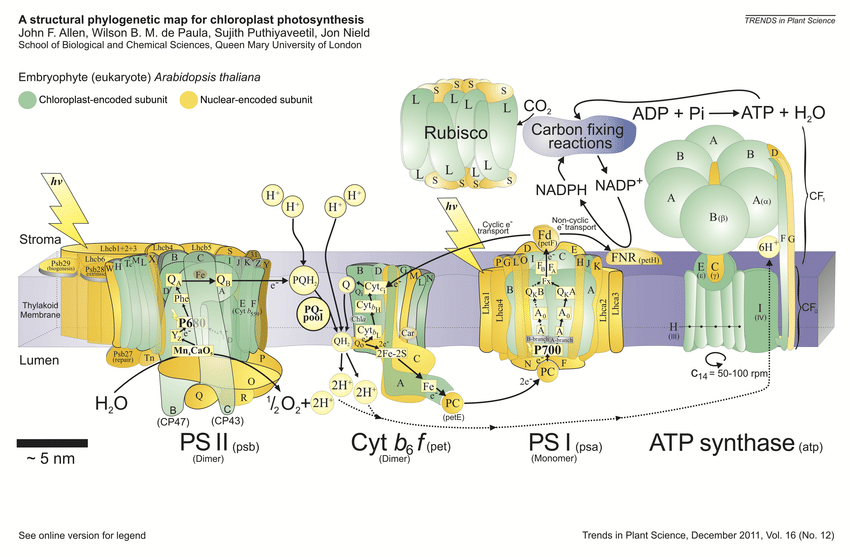
-
- A phenomenon known as quantum walk increases the efficiency of the energy transport of light significantly. In the photosynthetic cell of an alga, bacterium, or plant, there are light-sensitive molecules called chromophores arranged in an antenna-shaped structure named a photocomplex. When a photon is absorbed by a chromophore, it is converted into a quasiparticle referred to as an exciton, which jumps from chromophore to chromophore towards the reaction center of the photocomplex, a collection of molecules that traps its energy in a chemical form accessible to the cell’s metabolism. The exciton’s wave properties enable it to cover a wider area and try out several possible paths simultaneously, allowing it to instantaneously “choose” the most efficient route, where it will have the highest probability of arriving at its destination in the minimum possible time. Because that quantum walking takes place at temperatures far higher than quantum phenomena usually occur, it is only possible over very short distances.
Photosynthetic Pigments/Chromophores #
- A chromophore is the part of a molecule responsible for its color. The 11 double bonds in β-Carotene,
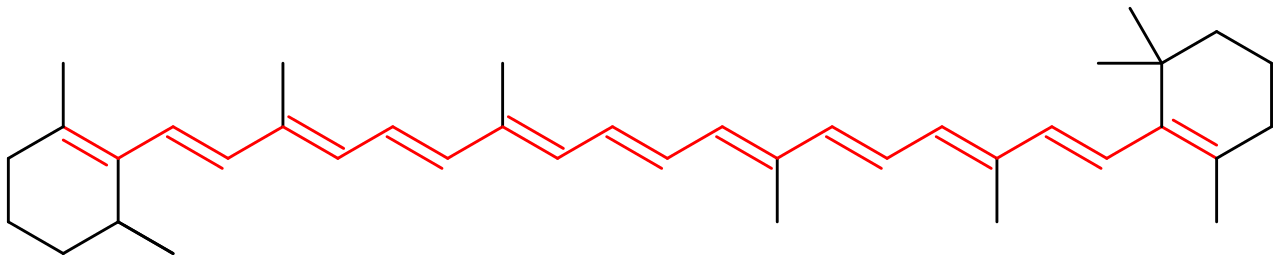 or in Porphyrin:
or in Porphyrin:
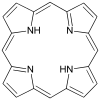 are responsible, somehow.
are responsible, somehow. -
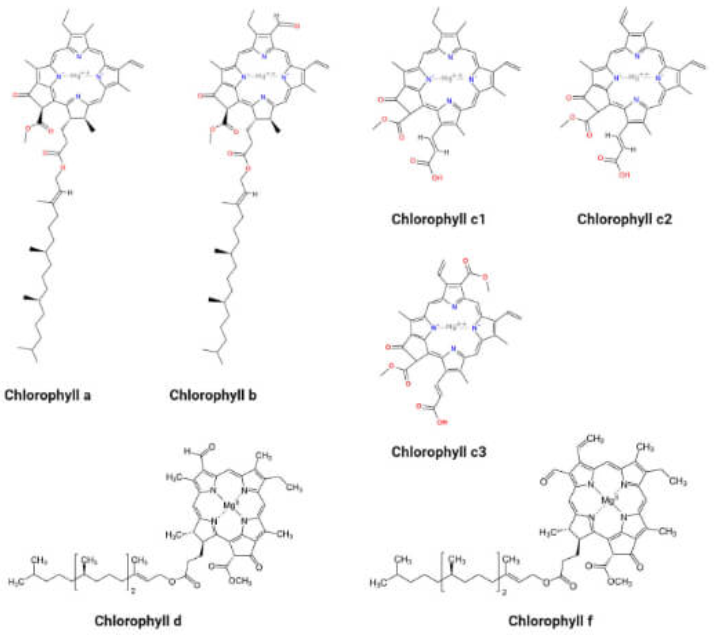
- Carotene: an orange pigment
- Xanthophyll: a yellow pigment
- 400–530
- Phaeophytin a: a gray-brown pigment
- Phaeophytin b: a yellow-brown pigment
- Chlorophyll a: a blue-green pigment (most common; present in every photosynthetic plant)
- Best absorption at 400–450 nm & 650–700 nm
- Chlorophyll b: a yellow-green pigment
- 450–500 nm & 600–650 nm
- Phycobilins (φύκος - alga; bilin are Porphyrin metabolites & pigments - including Bilirubin & Biliverdin)
- Phycoerythrobilin: red
- Phycourobilin: orange
- Phycoviolobilin (AKA phycobiliviolin) found in phycoerythrocyanin
- Phycocyanobilin (AKA phycobiliverdin): blue
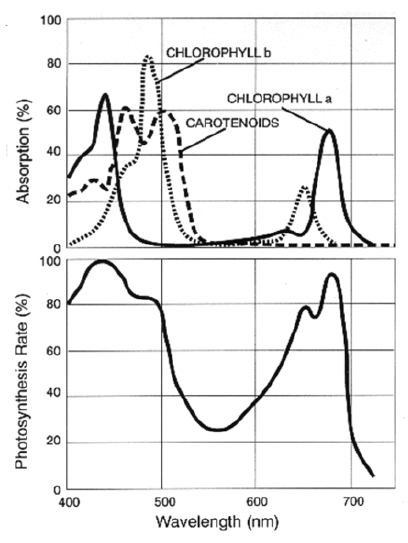
- That big dip in the 500-600 range is green-yellow. The question is why they take up red instead of green, when the latter is higher energy? The sun is on the oranger side and it tapers off:
-

- The answer is that it would actually be too much energy, and that the current chlorophyll-a type plants which populate the world may have somewhat arbitrarily outcompeted b-type, which is more blue.
-