Beyond the tilled plain, beyond the toy roofs, there would be a low suffusion of inutile loveliness, a low sun in a platinum haze with a warm, peeled-peach tinge pervading the upper edge of a two-dimensional, dove-grey cloud fusing with the distant amorous mist. There might be a line of spaced trees silhouetted against the horizon, and hot still noons above a wilderness of clover, and Claude Lorrain clouds inscribed remotely into misty azure with only their cumulus part conscpicuous against the neutral swoon of the background. Or again, it might be a stern El Greco horizon, pregnant with inky rain, and a passing glimpse of some mummy-necked farmer, and all around alternating strips of quick-silverish water and harsh green corn, the whole arrangement opening like a fan, somewhere in Kansas.
Color Vision
You can read the Wikipedia page on color blindness for a primer on what we’re about to get into.
 (This is not an accurate ’test’ per se for those with color blindness, and it is only a simulation. In reality it’s actually a bit difficult to simulate what the result of color blindness looks like, as it’s not like you just edit out a color channel from the image)
(This is not an accurate ’test’ per se for those with color blindness, and it is only a simulation. In reality it’s actually a bit difficult to simulate what the result of color blindness looks like, as it’s not like you just edit out a color channel from the image)
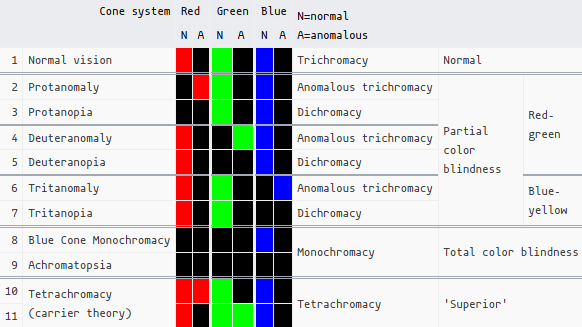
Essentially, in dichromacy, a gene for one of the opsins is absent. In anomalous trichromacy, all 3 opsins are present, but one of them has (a significant amount of) genetic mutations (a “hybrid gene”; an “L-M chimera”) that alters its spectral sensitivity (this is what those glasses excel at):
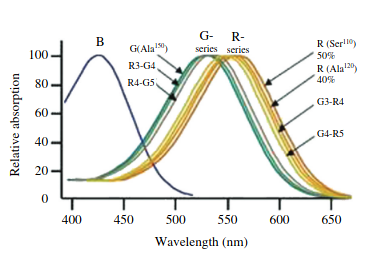 (From: The molecular basis of variation in human color vision)
(From: The molecular basis of variation in human color vision)
Color blindness is more common in males (5-8% of men vs. <1% in females) because OPN1LW/OPN1MW are found on the X chromosome (OPN1SW is on chromosome 7). Females, of course, have 2 X chromosomes, thus if one lacks the gene for an opsin, this would also make her a carrier of color blindness.
On the flip side, this is also somewhat the basis for theories about human tetrachromacy. Indeed, it’s probably only possible in females, such as when an additional, different OPN1LW/OPN1MW is somehow expressed.
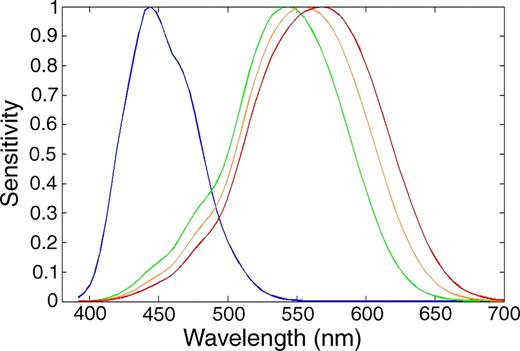
This genetic basis for this is actually rather common (~12%), but true ‘functional tetrachromacy’ is a rarity, and something that hasn’t been studied too extensively. In The dimensionality of color vision in carriers of anomalous trichromacy, they showed that 1 woman out of 24 with deuteranomaly (who thus are assumed to be retinal/non-functional tetrachromats) exhibited true tetrachromacy. The artist Concetta Antico is a living tetrachromat who’s gotten her fair share fo media attention. Apparently she has access to ~100 million colors, as opposed to ~1-10 million (conflicting sources…).
The Graphium Sarpedon, the bluebottle butterfly, has 15 different photoreceptors:
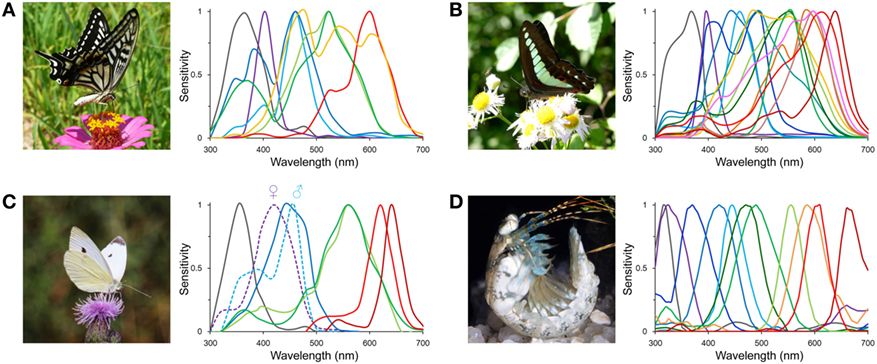 (From: The More, the Better? A Butterfly with 15 Kinds of Light Sensors in Its Eye)
(From: The More, the Better? A Butterfly with 15 Kinds of Light Sensors in Its Eye)
The mantis shrimp is famous for having 12 different photoreceptors, but unfortunately: Despite the impressive range of wavelengths that mantis shrimp have the ability to see, they do not have the ability to discriminate wavelengths less than 25 nm apart. It is suggested that not discriminating between closely positioned wavelengths allows these organisms to make determinations of its surroundings with little processing delay. Having little delay in evaluating surroundings is important for mantis shrimp, since they are territorial and frequently in combat. (Wikipedia)
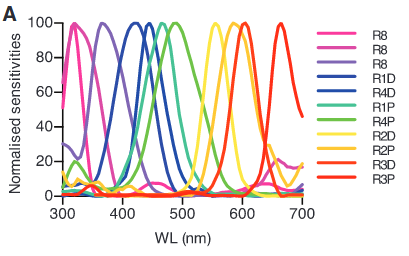 (From: A different form of color vision in mantis shrimp (2014): Theoretical approaches have predicted that between four and seven photoreceptor types are all that is needed to accurately encode the colors of the visible spectrum.)
(From: A different form of color vision in mantis shrimp (2014): Theoretical approaches have predicted that between four and seven photoreceptor types are all that is needed to accurately encode the colors of the visible spectrum.)
Avenues for Modification
Consider what occured in evolution: in humans and some other primates, OPN1LW branched off from OPN1MW as we escaped from the nocturnal bottleneck.
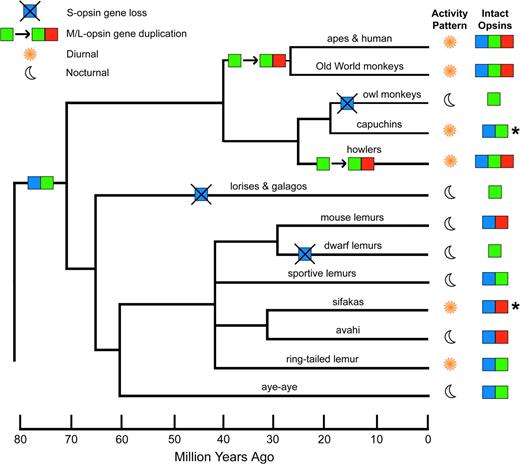 (Source)
(Source)
Or more subtle changes, like for instance, in Spectral-tuning mechanisms of marine mammal rhodopsins and correlations with foraging depth Fasick et al. describes how bottleneck dolphin rhodopsin blue-shifted during evolution (since it dives deep in the big blue) from an λmax of ~500 nm to 488 nm via a substitution of 3 amino acids.
Molecular determinants of human red/green color discrimination: The human red and green color vision pigments are identical at all but 15 of their 364 amino acids, and yet their absorption maxima differ by 31 nm… the spectral difference between these 2 pigments is shown to be determined by 7 and only 7 amino acid residues. For example, one of the residues is the Ser180Ala SNP (rs1557157655) on OPN1LW where it has a ~4-7 nm longer λmax. This SNP actually accounts for small differences in the general human population.
And to reiterate: as mentioned in the beginning, anomalous opsins are somewhere in between, with a few of these 7 residues differing. And, again: that’s part of what we see in human retinal tetrachromacy, where a 4th, anomalous cone that differs from its sister on the other X chromosome is added. Therefore, let’s design a whole gamut! Because now for the fun stuff.
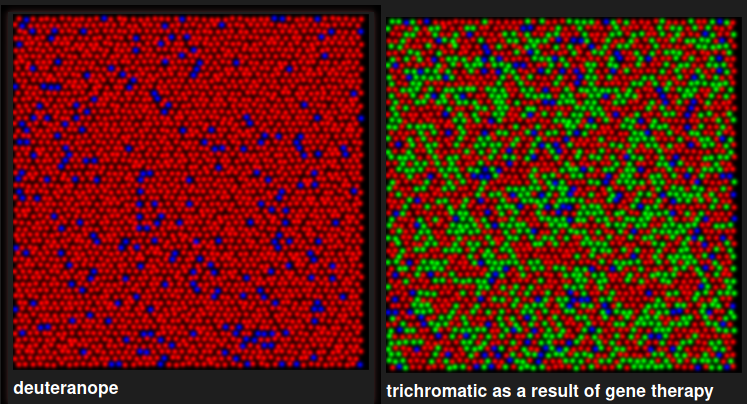
So back in 2009, The Neitz Lab cured color blindness(?) in squirrel monkeys, whose males (and some females) are natural dichromats:
-
Gene therapy for red-green colour blindness in adult primates (Manusco et al. 2009)
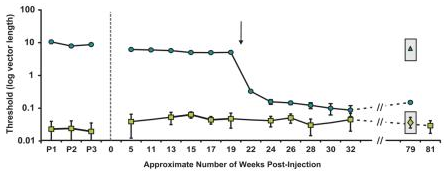
- As you can see in Fig. 3, it took an entire 20 weeks after the gene therapy for a sudden onset of color discrimination, which persisted at similar levels from 24 weeks onwards, after 2 years.
-
- As you can see in Fig. 3, it took an entire 20 weeks after the gene therapy for a sudden onset of color discrimination, which persisted at similar levels from 24 weeks onwards, after 2 years.
-
Curing color blindness–mice and nonhuman primates (Neitz & Neitz 2014)
Emergence of Novel Color Vision in Mice Engineered to Express a Human Cone Photopigment (2007)
Thing is, these are animals. We don’t actually have proof they’re perceiving red/green just like a trichromatic would. Perhaps it’s merely an enhanced sensitivity within the deuteranope spectrum somehow?
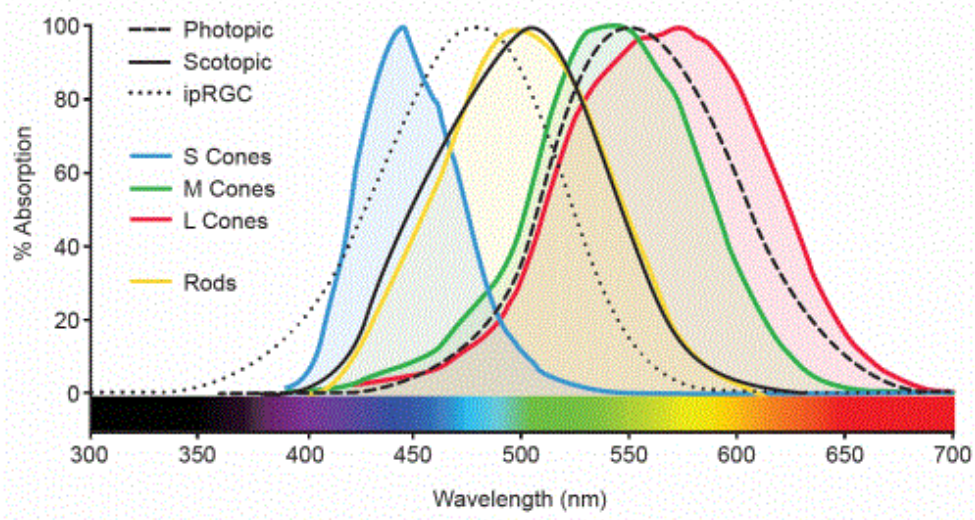
The Neitz Lab did their best. For instance, a hallmark of dichromatic vision is the neutral point, in the middle of the visible spectrum where the sensitivity of both cones overlaps and light appears achromatic. In deuteranopes this is ~500 nm, and ~495 for protanopes. Manusco et al. writes: As shown in Fig. 3b and c, both monkeys’ measured thresholds for these additional hues were similar to their thresholds for DW = 490 nm, demonstrating they now lacked a spectral neutral point and have become truly trichromatic. Furthermore, treated monkeys were able to discriminate blue-green (DW = 490 nm) when it was tested against a red-violet background (DW = −499 nm), instead of the grey background, indicating that the monkeys’ newly-acquired “green” and “red” percepts were distinct from one another.
Some concerns are further discussed in Is adding a new class of cones to the retina sufficient to cure color-blindness? (2015): we simulate how replacing the pigment of some cones is expected to influence the outcomes on the behavioral test used so far. The simulations show that this test does not provide conclusive evidence that the animals acquired the ability to make new chromatic distinctions. In our view, it is therefore premature to claim that human color-blindness can be cured through gene therapy. They also reference how, importantly, unlike other aspects of vision, color deprivation in early life does not preclude the proper development of color vision later in adulthood (e.g.: Striking absence of long-lasting effects of early color deprivation on monkey vision (1990))
Ultimately? Unless we figure out how to talk to monkeys about the nature of mind, we need better studies to give us more concrete inductive evidence, or we need to just try it in humans in order for us to really know how your vision changes and what the world looks like.
Furthermore, let us consider the physiology of cone cells more closely:
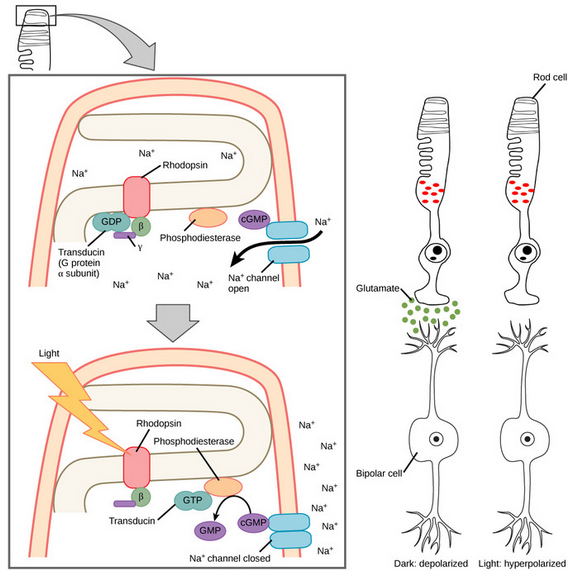 (These are rod cells, but cone cells are the exact same mechanism)
(These are rod cells, but cone cells are the exact same mechanism)
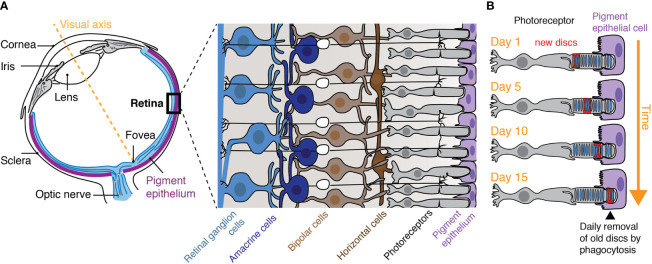 (From: Phagocytosis by the Retinal Pigment Epithelium: Recognition, Resolution, Recycling)
(From: Phagocytosis by the Retinal Pigment Epithelium: Recognition, Resolution, Recycling)
So the thing is, cone cells (unlike rod cells) aren’t continually replaced or anything during one’s lifetime, and each cell only expresses a single opsin gene; we can’t just replace the expression of a certain percentage of cone cells with another. As shown in the Neitz Lab study, the AAV encoding human L-opsin was targeted to M cones, leading to a coexpression of both types on the same cone cell (the resulting neural signaling being something rather difficult to wrap your head around?)
Perhaps even more interesting, there are currently human clinical trials underway for the treatment of achromatopsia—monochromacy, amongst other things. There are ~6 genes that can cause achromatopsia. Unlike monochromacy proper/cone monochromacy, achromatopsia is an abberation in the downstream phototransduction cascade. All 3 opsins are still present; the cells are dysfunctional.
Nonetheless, there are still some things we can learn from these cases, like the question of how the perception of color is acquired in the visual cortex, and things of that sort. Where are these people though? I do not know.
-
Cortical Visual Mapping following Ocular Gene Augmentation Therapy for Achromatopsia (2021)
-
A demonstration of cone function plasticity after gene therapy in achromatopsia (2022)
-
Seeing color following gene augmentation therapy in achromatopsia (2023) In humans.
- We suggest that treated CNGA3-achromatopsia patients can perceive a stimulus’s color attribute, although in a manner that is different and very limited compared with sighted individuals.
Gene Therapy for Color Blindness (2017)
And indeed, before there were human trials, the prerequisite treatment in animal models has also been demonstrated to be successful: AAV-Mediated Cone Rescue in a Naturally Occurring Mouse Model of CNGA3-Achromatopsia (2012), etc.
Beyond the Visible Spectrum
What about UV or infrared vision? What about X-ray vision, or maybe radio wave vision?
In cases of aphakia, such as with cataracts surgery (which Claude Monet had done in one eye! It gave his vision a bluish tint) prior to the invention of UV-blocking intraocular lenses in the 1980s, ultraviolet light (down to ~300 nm) is in fact visible—it’s the lens of the eye that filters out wavelenghts under ~400 nm. And to all 3 cones no less, leading to those with aphakia seeing UV as slightly bluish (since the S-cone is a bit more sensitive, as you may assume) white.
UV-sensitive tetrachromats, such as certain birds, do indeed have a cone specialized for detecting light in the UV range:
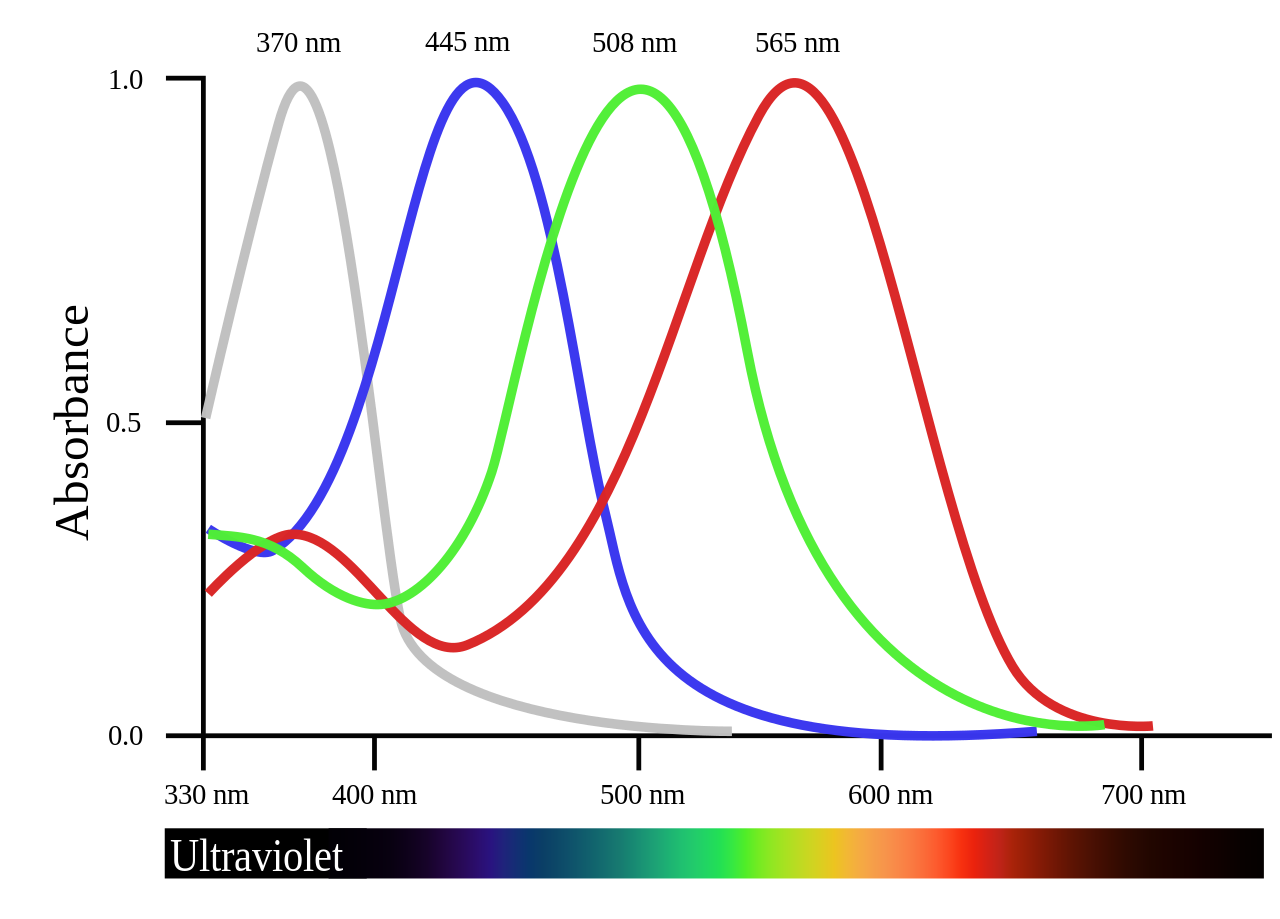
Perhaps the sequence for one of these SWS1 genes similar in homology to human OPN1SW that peaks in the UV range could be investigated for our purposes. cf. Evolution of color vision in primates - Evolutionary pathway of SWS1 - Wikipedia. Or, we could simply modify the existing amino acid sequence of human OPN1SW in order to shift its λmax further towards UV, as described in the previous section.
But the thing about UV light is that it’s not exactly healthy to your cells. It goes without saying neither is X-ray or gamma ray vision. The thing about animals with UV vision not facing negative repurcussions—it’s theorized they simply don’t live long enough to face the repurcussions of it.
As for infrared, as Wikipedia explains, there is no limit necessarily, just an exponential drop in sensitivity after 700 nm. Powerful NIR lasers are visible to the naked eye up to about 1050 nm, but that’s not exactly an everyday scenario.
Reason being, we’re limited by chemistry. A photon’s energy is proportional to its frequency: E = hν. Frequency is inversely related to wavelength, such that hν = hc/λ. And when we get to the infrared range, the energy is too low for 11-cis-retinal to be photoisomerized into all-trans retinal (this is an integral step of the phototransduction cascade, if the photon part didn’t make that obvious.)
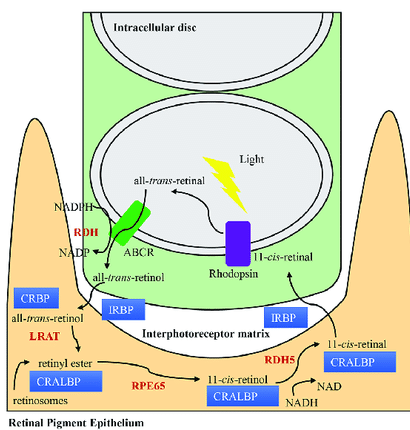 (From: The quest of vision: retina’s daily challenges)
(From: The quest of vision: retina’s daily challenges)
But interestingly, as Retina, Retinol, Retinal and the Natural History of Vitamin A as a Light Sensor explains: Although retinal is the universal chromophore for all vitamin A-based light sensors, the exact isomer of retinal can be different between species. For example, aquatic animals are known to shift absorption maxima of visual pigments by using the A1 (11-cis retinal) or A2 (11-cis-3,4-dehydroretinal) chromophore. There are also examples of terrestrial vertebrates using vitamin A2-based visual pigments, which belong to the most red-shifted visual pigments (e.g., absorption maximum of 625 nm). A2 pigments absorb longer wavelengths of light compared to the A1 version because of the extension of the conjugated chain of the chromophore.
Maybe just maybe, if amino acid structure of opsins is not the bottleneck for making the deepest red cone possible, we could benefit from squeezing out some red sensitivity by making the switch to vitamin A2 (which in humans is produced from al-trans-retinol via CYP27C1), and also making sure we support the metabolism of it. But… it’s pretty minute difference:
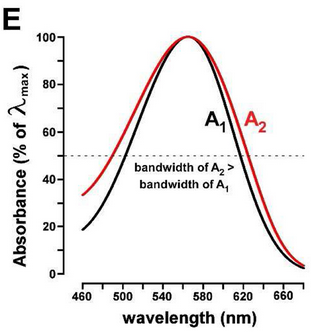 (From: Vitamin A1/A2 chromophore exchange: Its role in spectral tuning and visual plasticity)
(From: Vitamin A1/A2 chromophore exchange: Its role in spectral tuning and visual plasticity)
Replacement of the chromophore has been done in vitro at least: Chromophore switch from 11-cis-dehydroretinal (A2) to 11-cis-retinal (A1) decreases dark noise in salamander red rods
Eye Color
WIP section, because honestly I’m not sure by what means the pigmentation of the iris would actually be meaningfully altered after initially being created, since I don’t believe there’s any sort of continuous renewal process.
This one’s just for fun, especially since it should be pretty easy as far as gene editing goes, with no complicated biochemical mechanism we’re altering here—just flipping some SNPs. That does require CRISPR in this case, rather than AAV. And, as we’ll discuss in the next section, I think it’s possible this may even be doable with eye drops.
Well, Wikipedia has a number of references for the matter. OCA2 and HERC2 are the main genes. But there are hundreds of SNPs. And of course a wide array of genes for other pigments.
In albinism, the iris is actually able to become red (not a good look—especially in more severe cases where the pupil is also completely red—but to each their own) or in less severe cases, violet, which when the iris is decorated with eye-catching lacunae, and a contrasting (read: pigmented) limbal ring, I find to be quite attractive—at least in the, like, 3 different people you see when you look up “albino purple eyes”. So maybe it’s something you could shoot for. The actress Elizabeth Taylor was famous for haing deep blue eyes that looked violet in certain lighting conditions (and also having distichiasis, double eyelashes.)
Gene Therapy (Do-It-Yourself?)
Is retinal gene therapy via eyedrops possible? For our purposes I’m skeptical, but who knows. Fistly, we ought to only bring our gene vector into the retina and nowhere else. Don’t ask me what would even happen if cones started to be expressed in areas they shouldn’t (my guess is that that’s highly unlikely), or if they would even survive or integrate with anything, etc.. With eyedrops, the drug obviously first enters the cornea, conjunctiva, lens, iris, etc. with little of it reaching the postior segment (Recent Perspectives in Ocular Drug Delivery (2015) - PMC) owing to the series of barriers before reaching the cone cells in the retina.
There was indeed a case where HSV-1 was delivered via eyedrops and successfully cured a boy’s blindness related to dystrophic epidermolysis bullosa, where type VII collagen formation is disrupted—which exists in epithelial regions of the eye—not the retina (and, you know, only the retina).
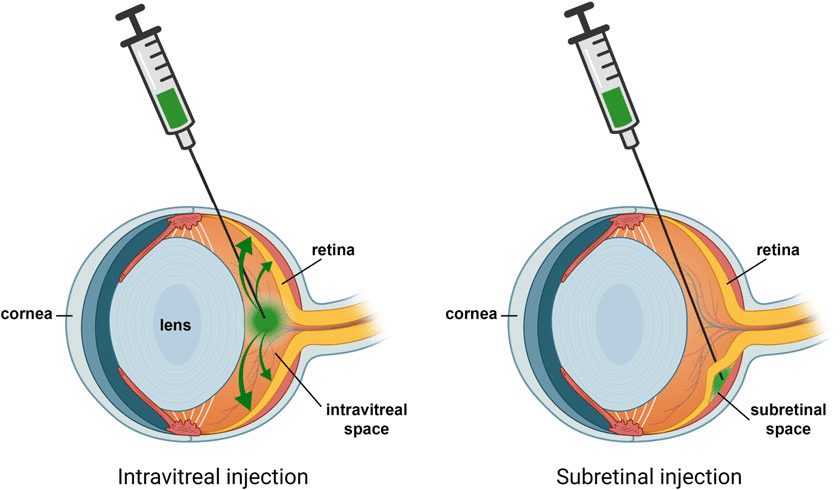
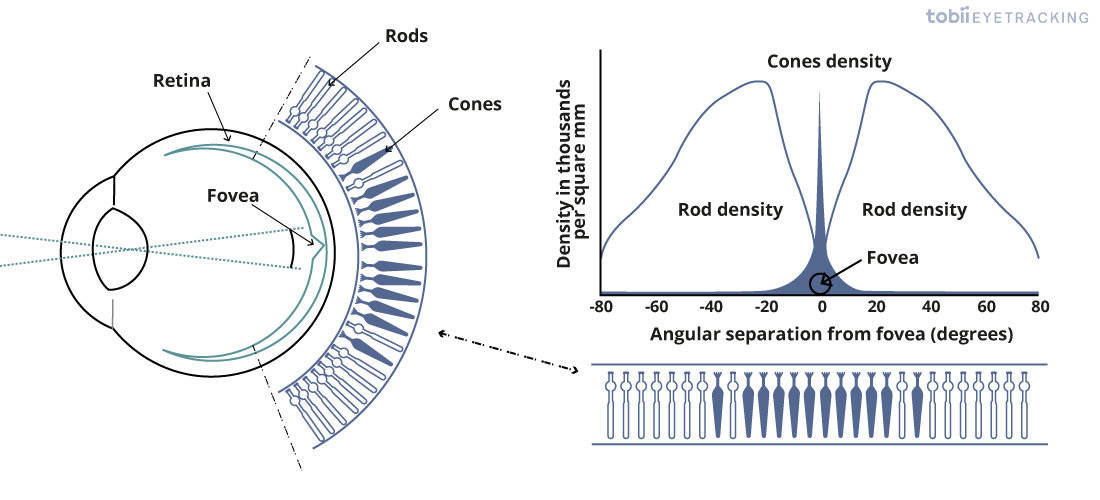
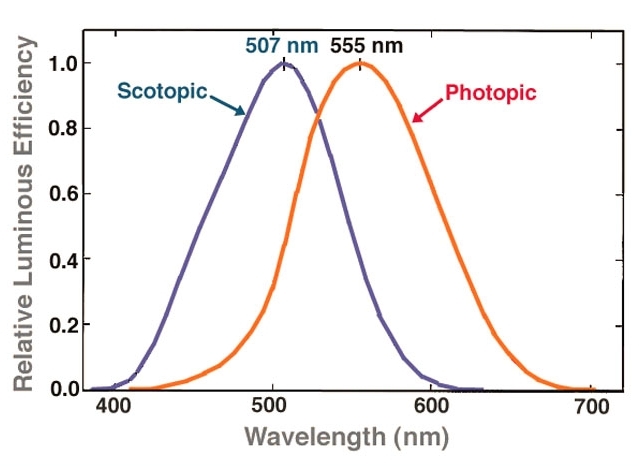
Even intravitreal injection could be too diffusive in that regard. But take the case of treating retinal vein occlusion: the central retinal vein is located in the most postior segment of the eye, and anti-VEGF drugs are injected intravitreally (whereby it diffuses evenly to the entire retina) there, in order to counteract neovascularization/edema/etc. in certain conditions like macular degeneration. However: The Neitz lab in Efficiency of gene therapy via intravitreal injection in primate cones (2022) and Poster Session II: Intravitreal gene therapy in primate reaches extrafoveal cones (2023) showed that intravitreal AAV can indeed reach the retina and the fovea. And reaching the fovea/macula lutea is definitely important for our purposes: essentially it is the region of the eye necessary for detailed, photopic vision, and it is where cone cells are most densely concentrated (especially M- and L-cones, as done in this study), with no rods present, as opposed to the high density of rod cells in the periphery of the retina:
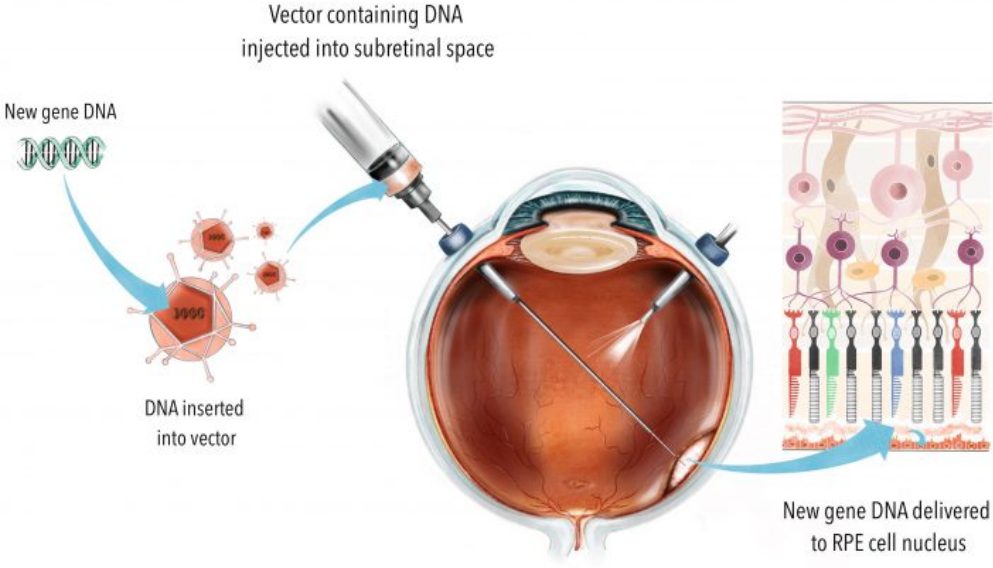
The more invasive and complex subretinal injection is how Manusco et al. delivered their AAV into squirrel monkeys (via 3 separate injections, along with a cocktail of other drugs so that things went smoothly), and it’s how other retinal gene therapies (For example, Luxturna®/Voretigene neparvovec, which obtained FDA approval in 2017) are being performed: Subretinal Injection Techniques for Retinal Disease: A Review, Gene therapy in retinal diseases: A review (2021).
Now performing intravitreal injection of a genetically engineered virus into your own eyeballs is not something I recommend you do at home. But, I will note that in theory, a great deal of genetic engineering (say injecting something intramuscularly instead) is literally a thing you can do at home. I’ll link a few things that explain the process better than I can:
The YouTube channel The Thought Emporium has a series Whose Gene is it Anyway where he constructs an exact plan/shopping list for knocking in/out certain genes in plants or in vitro cells. You may have seen his video I Genetically Engineered MYSELF to Fix Lactose Intolerance where he makes his microvilli express E. coli lactase, via AAV, delivered orally in microcrystalline cellulose pills. A single dose made him fully lactose tolerant for 12 months before the benefits started to even slightly fade.
Why this and similar potential therapies aren’t well-known and more ubiquitously used is beyond me and it’s proof the general public is way behind science. It’s like when I realized jetpacks aren’t science fiction one time when I was a kid. What’s even less obvious to some is that genetic engineering isn’t even exactly cutting-edge technology. It was achieved as early as the 1970s (like a number of other great things). Such a profoundly beneficial technology (~2-3% of human diseases are single-gene disorders—not bad) has gone under the radar probably due to bullshit biology reasons, since I guess things are easier said than done. But once you realize how easily things are in fact said, it’s hard to see why we’re not seeing larger mass adoption.
As far as I know, price (and legality, but that’s what Honduras is for) is the main hang-up when it comes to visiting gene therapy clinics. How much it is just mark up and some initial intellectual labor? Probably most of it. Some of these treatments such as Hemgenix®/Etranacogene dezaparvovec (intravenous AAV delivery of Factor IX), while sometimes covered by medical insurance (look how they did Shkreli?) can cost illions.
Remember that some of this is theoretically able to be done DIY, obtaining plasmids and the like simply by ordering them online. You just might have to find a cartel opthamologist.
You can watch the 4-episode TV series Unnatural Selection to get a better portrait of the ‘genetic biohacker’ spirit I’m talking about. Amongst other people, they follow Aaron Traywick (who was unfortunately later found dead in a sensory deprivation tank in 2018 with ketamine in his system). His views on gene editing erred on the anarchistic side; something that could have led us all into a very interesting timeline. He had grandiose (but one must wonder if they weren’t realistic, and benevolent) aspirations on curing an array of diseases such as HIV and AIDS via gene editing. They also cover some members of the crew at The Odin (Jo Zayner, David Ishee, et al.) who share a similar sentiment. David Ishee is one of the few people/biohackers so far to undergo (self-administered!) gene therapy for ‘designer’ purposes while keeping the public in the loop: increasing follistatin levels. This is a pretty well-studied therapy in the longevity field (that includes in humans, for muscular dystrophy and the like) compared to the millions of other things one could do, so I see the rationale. He successfully did it in mice first, and then on himself, but ultimately with questionable results (at least in terms of the effect on his physique, IIRC?) for reasons unknown. Bryan Johnson recently joined the ranks, and before that there were people like Liz Parrish, who has received gene therapy for increasing follistatin, klotho, and telomerase. Or Brian Hanley, who gave himself an extra copy of GHRH as proof-of-concept for the treatment of AIDS—however that works.